Bicarbonates
 |
|
 |
|
| Names | |
|---|---|
|
Systematic IUPAC name
Hydroxidodioxidocarbonate(1−)
|
|
| Other names
Hydrogencarbonate
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| 3DMet | B00080 |
| 3903504 | |
| ChEBI | |
| ChemSpider | |
| 49249 | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| HCO− 3 |
|
| Molar mass | 61.0168 g mol−1 |
| log P | −0.82 |
| Acidity (pKa) | 10.3 (Conjugate acid of carbonate) |
| Basicity (pKb) | 7.7 (Conjugate base of carbonic acid) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogen carbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.
Bicarbonate serves a crucial biochemical role in the physiological pH buffering system.
The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The prefix "bi" in "bicarbonate" comes from an outdated naming system and is based on the observation that there is twice as much carbonate (CO2−
3) per sodium ion in sodium bicarbonate (NaHCO3) and other bicarbonates than in sodium carbonate (Na2CO3) and other carbonates. The name lives on as a trivial name.
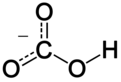
The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula HCO−
3 and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid HNO
3. The bicarbonate ion carries a negative one formal charge and is the conjugate base of carbonic acid H
2CO
3; at the same time, it is the conjugate acid of CO2−
3, the carbonate ion, as shown by these equilibrium reactions:
...
Wikipedia
