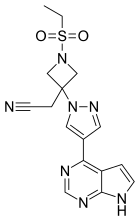
Baricitinib
 |
|
| Clinical data | |
|---|---|
| Trade names | Olumiant |
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| Synonyms | INCB28050, LY3009104 |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.219.080 |
| Chemical and physical data | |
| Formula | C16H17N7O2S |
| Molar mass | 371.42 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Baricitinib (trade name Olumiant) is an investigational drug for rheumatoid arthritis (RA), being developed by Incyte and Eli Lilly. It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.
In January 2016, Eli Lilly submitted a new drug application to the US FDA for the approval of baricitinib to treat moderately-to-severely active RA.
In December 2016, the European Committee for Medicinal Products for Human Use (CHMP) recommended the approval of baricitinib as a second-line therapy for RA in adults, either alone or in combination with methotrexate.
Despite widespread expectations that the FDA would approve baricitinib for RA, in April 2017, the FDA issued a rejection, citing concerns about dosing and safety.
Other JAK inhibitors include tofacitinib, currently approved for the treatment of RA, and ruxolitinib.
...
Wikipedia
