Barbitone
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682221 |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 30.3 (± 3.2) hours |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.301 |
| Chemical and physical data | |
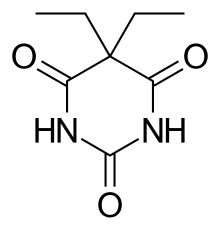
| Formula | C8H12N2O3 |
| Molar mass | 184.193 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Barbital (as known in the United States) or barbitone (as known elsewhere), marketed under the brand names Veronal for the pure acid and Medinal for the sodium salt, was the first commercially available barbiturate. It was used as a sleeping aid (hypnotic) from 1903 until the mid-1950s. The chemical names for barbitone are diethylmalonyl urea or diethylbarbituric acid; hence, the sodium salt (known as medinal, a genericised trademark in the United Kingdom) is known also as sodium diethylbarbiturate.
Barbitone was first synthesized in 1902 by German chemists Emil Fischer and Joseph von Mering, who published their discovery in 1903. Barbitone was prepared by condensing diethylmalonic ester with urea in the presence of sodium ethoxide, and then by adding at least two molar equivalents of ethyl iodide to the silver salt of malonylurea or possibly to a basic solution of the acid. The result was an odorless, slightly bitter, white crystalline powder.
Barbitone can also be synthesized in a condensation reaction from urea and diethyl-2,2-diethylmalonate, a diethyl malonate derivative:
Barbitone was marketed in 1904 by the Bayer company as “Veronal”. A soluble salt of barbitone was marketed by the Schering company as “Medinal.” It was dispensed for “insomnia induced by nervous excitability”. It was provided in either capsules or cachets. The therapeutic dose was ten to fifteen grains (0.65-0.97 grams). 3.5 to 4.4 grams is the deadly dose but sleep has also been prolonged up to ten days with recovery.
Barbitone was considered to be a great improvement over the existing hypnotics. Its taste was slightly bitter, but better than the strong, unpleasant taste of the commonly used bromides. It had few side effects, and its therapeutic dose was far below the toxic dose. However, prolonged usage resulted in tolerance to the drug, requiring higher doses to reach the desired effect. "I'm literally saturated with it," the Russian tsarina Alexandra Feodeorovna confessed to a friend. Fatal overdoses of this slow-acting hypnotic were not uncommon. Pioneering aviator Arthur Whitten Brown (of "Alcock and Brown" fame) died of an accidental overdose. Japanese writer Ryūnosuke Akutagawa deliberately overdosed on the drug in 1927.
...
Wikipedia
