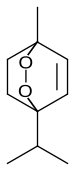
Ascaridole
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
1-Methyl-4-(1-methylethyl)-2,3-dioxabicyclo[2.2.2]oct-5-ene
|
|||
| Identifiers | |||
|
512-85-6 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:2866 |
||
| ChEMBL |
ChEMBL467614 |
||
| ChemSpider |
10105 |
||
| ECHA InfoCard | 100.007.408 | ||
| PubChem | 10545 | ||
|
|||
|
|||
| Properties | |||
| C10H16O2 | |||
| Molar mass | 168.23 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.010 g/cm3 | ||
| Melting point | 3.3 °C (37.9 °F; 276.4 K) | ||
| Boiling point | 40 °C (104 °F; 313 K) at 0.2 mmHg | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Ascaridole is a natural organic compound classified as a bicyclic monoterpene that has an unusual bridging peroxide functional group. It is a colorless liquid with a pungent smell and taste that is soluble in most organic solvents. Like other low molecular weight organic peroxides, it is unstable and prone to explosion when heated or treated with organic acids. Ascaridole determines the specific flavor of the Chilean tree boldo and is a major constituent of the oil of Mexican tea (wormseed). It is a component of natural medicine, tonic drinks and food flavoring in Latin American cuisine. As part of the oil, ascaridole is used as an anthelmintic drug that expels parasitic worms from plants, domestic animals and the human body.
Ascaridole was the first, and for a long time only, discovered naturally occurring organic peroxide. It was isolated from Chenopodium oil and named by Hüthig in 1908, who described its explosive character and determined its chemical formula as C10H16O2. Hüthig also noted the indifference of ascaridole to aldehydes, ketones or phenols that characterized it as non-alcohol. When reacted with sulfuric acid, or reduced with zinc powder and acetic acid, ascaridole formed cymene. These results were confirmed in a detailed study by E. K. Nelson in 1911, in particular that ascaridole explodes upon heating, reacting with sulfuric, hydrochloric, nitric, or phosphoric acids. Nelson showed that the new substance contained neither a hydroxyl nor a carbonyl group and that upon reduction with iron(II) sulfate it formed a glycol, now known as ascaridole glycol, C10H18O3. The glycol is more stable than ascaridole and has a higher melting point of about 64 °C, boiling point of 272 °C, and density of 1.098 g/cm3. Nelson also predicted the chemical structure of ascaridole which was almost correct, but had the peroxide bridge not along the molecular axis, but between the other, off-axis carbon atoms. This structure was corrected by Otto Wallach in 1912.
...
Wikipedia