Arsenic trisulfide
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Arsenic trisulfide
|
|
| Other names
Arsenic(III) sulfide
Orpiment |
|
| Identifiers | |
|
1303-33-9 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
21241348 |
| ECHA InfoCard | 100.013.744 |
| EC Number | 215-117-4 |
| PubChem | 4093503 |
| RTECS number | CG2638000 |
| UNII |
44SIJ800OX |
|
|
|
|
| Properties | |
| As2S3 | |
| Molar mass | 246.02 g·mol−1 |
| Appearance | Orange crystals |
| Density | 3.43 g cm−3 |
| Melting point | 310 °C (590 °F; 583 K) |
| Boiling point | 707 °C (1,305 °F; 980 K) |
| -70.0·10−6 cm3/mol | |
| Structure | |
| P21/n (No. 11) | |
|
a = 1147.5(5) pm, b = 957.7(4) pm, c = 425.6(2) pm
α = 90°, β = 90.68(8)°, γ = 90°
|
|
| pyramidal (As) | |
| Hazards | |
| GHS pictograms |
 
|
| GHS signal word | DANGER |
| H300, H331, H400, H411 | |
| NFPA 704 | |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3 |
|
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute] |
|
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)] |
| Related compounds | |
|
Other anions
|
Arsenic trioxide Arsenic triselenide |
|
Other cations
|
Phosphorus trisulfide Antimony trisulfide Bismuth sulfide |
|
Related compounds
|
Tetraarsenic tetrasulfide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
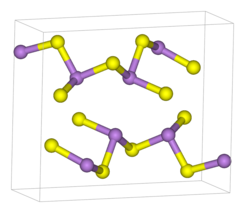
Orpiment
Arsenic trisulfide is the inorganic compound with the formula As2S3. It is a bright yellow solid that is insoluble in water. It also occurs as the mineral orpiment (Latin: auripigment), which has been used as a pigment called King's yellow. It is produced in the analysis of arsenic compounds. It is a group V/VI, intrinsic p-type semiconductor and exhibits photo-induced phase-change properties. The other principal arsenic sulfide is As4S4, a red-orange solid known as the mineral realgar.
As2S3 occurs both in crystalline and amorphous forms. Both forms feature polymeric structures consisting of trigonal pyramidal As(III) centres linked by sulfide centres. The sulfide centres are two-fold coordinated to two arsenic atoms. In the crystalline form, the compound adopts a ruffled sheet structure. The bonding between the sheets consists of van der Waals forces. The crystalline form is usually found in geological samples. Amorphous As2S3 does not possess a layered structure but is more highly cross-linked. Like other glasses, there is no medium or long-range order, but the first co-ordination sphere is well defined. As2S3 is a good glass former and exhibits a wide glass-forming region in its phase diagram.
Amorphous As2S3 is obtained via the fusion of the elements at 390 °C. Rapid cooling of the reaction melt gives a glass. The reaction can be represented with the chemical equation:
As2S3 forms when aqueous solutions containing As(III) are treated with H2S. Arsenic was in the past analyzed and assayed by this reaction, which results in the precipitation of As2S3, which is then weighed. As2S3 can even be precipitated in 6M HCl. As2S3 is so insoluble that it is not toxic.
Upon heating in a vacuum, polymeric As2S3 "cracks" to give a mixture of molecular species, including molecular As4S6. As4S6 adopts the adamantane geometry, like that observed for P4O6 and As4O6. When a film of this material is exposed to an external energy source such as thermal energy (via thermal annealing ), electromagnetic radiation (i.e. UV lamps, lasers, electron beams)), As4S6 polymerizes:
...
Wikipedia

