Arsenic acid
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Arsenic acid, arsoric acid
|
|
| Other names
Arsenic acid
Orthoarsenic acid Desiccant L-10 Zotox |
|
| Identifiers | |
|
7778-39-4 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:18231 |
| ChemSpider |
229 |
| ECHA InfoCard | 100.029.001 |
| EC Number | 231-901-9 |
| KEGG |
C01478 |
| RTECS number | CG0700000 |
| UNII |
N7CIZ75ZPN |
|
|
|
|
| Properties | |
| H3AsO4 | |
| Molar mass | 141.94 g/mol |
| Appearance | White translucent crystals, hygroscopic. |
| Density | 2.5 g/cm3 |
| Melting point | 35.5 °C (95.9 °F; 308.6 K) |
| Boiling point | 120 °C (248 °F; 393 K) decomposes |
| 16.7 g/100 mL | |
| Solubility | soluble in alcohol |
| Vapor pressure | 55 hPa (50 °C) |
| Acidity (pKa) | 2.19, 6.94, 11.5 |
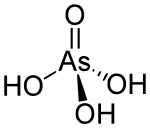
| Structure | |
| Tetrahedral | |
| Hazards | |
|
EU classification (DSD)
|
Toxic (T) Dangerous for the environment (N) |
| R-phrases | R23/25, R45, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
48 mg/kg (rat, oral) |
| Related compounds | |
|
Other anions
|
Phosphoric acid |
|
Other cations
|
Sodium arsenate |
|
Related compounds
|
Arsenous acid Arsenic pentoxide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Arsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it is largely ionized. Its hemihydrate form (H3AsO4·½H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.
It is a tetrahedral species of idealized symmetry C3v with As–O bonds lengths ranging from 1.66 to 1.71 Å.
Being a triprotic acid, its acidity is described by three equilibria:
These Ka values are close to those for phosphoric acid. The highly basic arsenate ion (AsO3−
4) is the product of the third ionization. Unlike phosphoric acid, arsenic acid is an oxidizer, illustrated by its ability to convert iodide to iodine.
Arsenic acid is prepared by treating arsenic trioxide with concentrated nitric acid and dinitrogen trioxide is produced as a by-product.
The resulting solution is cooled to give colourless crystals of the hemihydrate H3AsO4·½H2O, although the dihydrate H3AsO4·2H2O is produced when crystallisation occurs at lower temperatures.
Arsenic acid is slowly formed when arsenic pentoxide is dissolved in water, and when meta- or pyroarsenic acid is treated with cold water. Arsenic acid can also be prepared directly from elemental arsenic by moistening it and treating with ozone.
Commercial applications of arsenic acid are limited by its toxicity. It has found occasional use as a wood preservative, broad-spectrum biocide, a finishing agent for glass and metal, and a reagent in the synthesis of some dyestuffs and organic arsenic compounds. The LD50 in rabbits is 6 mg/kg (0.006 g/kg).
...
Wikipedia

