Amyloid-beta
| Amyloid beta peptide (beta-APP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|

A partially folded structure of amyloid beta(1 40) in an aqueous environment (pdb 2lfm)
|
|||||||||
| Identifiers | |||||||||
| Symbol | APP | ||||||||
| Pfam | PF03494 | ||||||||
| InterPro | IPR013803 | ||||||||
| SCOP | 2lfm | ||||||||
| SUPERFAMILY | 2lfm | ||||||||
| TCDB | 1.C.50 | ||||||||
| OPM superfamily | 369 | ||||||||
| OPM protein | 2y3k | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease) | |
|---|---|
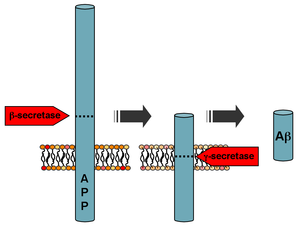
Processing of the amyloid precursor protein
|
|
| Identifiers | |
| Symbol | APP |
| Alt. symbols | AD1 |
| Entrez | 351 |
| HUGO | 620 |
| OMIM | 104760 |
| RefSeq | NM_000484 |
| UniProt | P05067 |
| Other data | |
| Locus | Chr. 21 q21.2 |
Amyloid beta (Aβ or Abeta) denotes peptides of 36–43 amino acids that are crucially involved in Alzheimer's disease as the main component of the amyloid plaques found in the brains of Alzheimer patients. The peptides derive from the amyloid precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers (known as "seeds") can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The seeds or the resulting amyloid plaques are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.
A recent study suggested that APP and its amyloid potential is of ancient origins, dating as far back as early deuterostomes.
The normal function of Aβ is not well understood. Though some animal studies have shown that the absence of Aβ does not lead to any obvious loss of physiological function, several potential activities have been discovered for Aβ, including activation of kinase enzymes, protection against oxidative stress, regulation of cholesterol transport, functioning as a transcription factor, and anti-microbial activity (potentially associated with Aβ's pro-inflammatory activity).
The glymphatic system clears metabolic waste from the mammalian brain, and in particular beta amyloids. The rate of removal is significantly increased during sleep. However, the significance of the glymphatic system is in Aβ clearance in Alzheimer's disease is unknown.
...
Wikipedia
