Amoebic liver abscesses
| Amoebic liver abscess | |
|---|---|
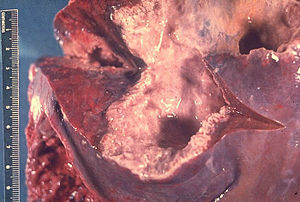 |
|
| Gross pathology of liver containing amoebic abscess | |
| Classification and external resources | |
| Specialty | infectious disease |
| ICD-10 | A06.4 |
| ICD-9-CM | 006.3 |
| MedlinePlus | 000211 |
| eMedicine | article/183920 |
| MeSH | D008101 |
A amoebic liver abscess is a type of liver abscess caused by amebiasis. It is the involvement of liver tissue by trophozoites of the organism Entamoeba histolytica and of is abscess due to necrosis.
Symptoms
Signs
Treatment must also include a lumenal amoebicide to prevent reinvasion of tissues by amoebae still in the intestines (see Amoebiasis). After completion of treatment with tissue amebicides, administer luminal amebicides for eradication of the asymptomatic colonization state. Failure to use luminal agents can lead to relapse of infection in approximately 10% of patients.
The treatment of invasive amoebiasis should be directed to all sites where E. histolytica may be present. Hence the ideal amoebicide should be able to act within the intestinal lumen, in the intestinal wall, and systemically, particularly in the liver. Systemic amoebicidal drugs include emetine, dehydroemetine, chloroquine diphosphate, metronidazole, and tinidazole.
Ipecac or ipecacuanha consists of the dried rhizome and roots of Cephaelis ipecacuanha. The medical virtues of ipecac are almost entirely due to the action of its alkaloids-emetine and cephaline. Till today, emetine remains one of the best drugs for treating amoebic liver abscess. It has a direct action on the trophozoites. Its greater concentration and duration of action in the liver as compared to that in the intestinal wall explains its high efficacy in amoebic liver abscess and also its low parasitic cure rate for intestinal amoebiasis. The drug is detoxicated and eliminated slowly. It may, therefore, produce cumulative effects. In man, emetine poisoning is characterized by muscular tremors, weakness and pain in the extremities which tend to persist until drug administration is stopped. Gastro-intestinal symptoms include nausea, vomiting and bloody diarrhoea. The latter may be mistaken for a recurrence of amoebic dysentery. Many clinicians fear the occurrence of cardiac toxicity due to this drug and hence avoid using it. Serious cardiac toxicity, however, is rare. Both recovered with the treatment for heart failure and withdrawal of emetine. One patient who was given fifteen injections of emetine in a dose of 60 mgm per day, died. Overdosage of emetine produces focal necrosis of cardiac muscle resulting in cardiac failure and sudden death. Emetine, like digitalis may produce mild ST and T wave changes in the electrocardiogram which does not necessarily mean serious toxicity. In fact, they are encountered, though less commonly, after the use of chloroquine and metronidazole as well. Toxic effects on the myocardium have been described even in doses generally considered safe. These are rise in pulse rate, fall in systolic blood pressure and ST-T changes in the electrocardiogram. The other rare E.C.G. changes include deformity of QRS complexes, prolongation of PR interval, atrial premature beats, and atrial tachycardia. In adults, fatal cases have been reported with a total dose of 0.6 G. or less. The incidence of toxic heart damage greatly increases in patients with anaemia. In patients having myocardial disease or marked hypertension, emetine can be used for amoebic liver abscess, as the benefits from it may outweigh possible hazards. This situation is unlikely to arise these days, as equally good alternative drugs like metronidazole are available. Patients receiving emetine should be monitored for changes in pulse, blood pressure and electrocardiography. Absolute bed rest during and several days after emetine therapy has been recommended, although we have often seen patients in whom no untoward reactions have occurred in spite of neglecting the above precaution. Theoretically the use of emetine in children is not advised. However, in practice it has been used as discussed elsewhere. It should not be administered during pregnancy unless absolutely necessary. Although emetine is undeniably moderately toxic, the risk of using it would be worth accepting in such a serious illness were it not for the fact that less toxic drugs like chloroquine and metronidazole are now available. In practice, emetine still produces a more dramatic clinical response thanchloroquine or metronidazole. This point would score in favour of emetine in places where facilities for a proper diagnosis are not available and a therapeutic test remains as the only weapon with a practitioner. Emetine should always be given deep intramuscularly or deep subcutaneously but never intravenously. The total dose in amoebic liver abscess should not exceed 650 mg or 10 mg/kg. This should be given over a period of 10 days in a dose of 6G65 mg. daily. A relapse rate of 7% follows one such course. Therefore, the treatment could be repeated after a period of 2–6 weeks. Of late such a need does not arise, as drug combinations are commonly used. When parenteral emetine is combined with oral chloroquine or two courses of emetine are given, the relapse rate can be brought down to 1 percent.
...
Wikipedia
