Ammonium salt
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
Ammonium
|
|||
| Identifiers | |||
| 14798-03-9 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:CHEBI:28938 | ||
| ChemSpider | 218 | ||
| MeSH | D000644 | ||
| PubChem | 16741146 | ||
|
|||
|
|||
| Properties | |||
| NH+ 4 |
|||
| Molar mass | 18.04 g·mol−1 | ||
| Acidity (pKa) | 9.25 | ||
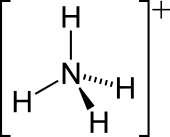
| Structure | |||
| Tetrahedral | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+
4. It is formed by the protonation of ammonia (NH3). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (NR+
4), where one or more hydrogen atoms are replaced by organic groups (indicated by R).
The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors):
The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule:
Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:
The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high (the concentration of hydrogen ions is low), the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia.
Formation of ammonium compounds can also occur in the vapor phase; for example, when ammonia vapor comes in contact with hydrogen chloride vapor, a white cloud of ammonium chloride forms, which eventually settles out as a solid in a thin white layer on surfaces.
...
Wikipedia


