Aluminium hydride
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Aluminium hydride
|
|
|
Systematic IUPAC name
Alumane
|
|
| Other names
Alane
Aluminic hydride |
|
| Identifiers | |
|
7784-21-6 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:30136 |
| ChemSpider |
13833 17625618 (3H3) |
| ECHA InfoCard | 100.029.139 |
| 245 | |
| PubChem |
14488 14399066 (2H3) 16721258 (3H3) |
|
|
|
|
| Properties | |
| AlH3 | |
| Molar mass | 29.99 g/mol |
| Appearance | white crystalline solid, non-volatile, highly polymerized, needle-like crystals |
| Density | 1.477 g/cm3, solid |
| Melting point | 150 °C (302 °F; 423 K) starts decomposing at 105 °C (221 °F) |
| reacts | |
| Solubility | soluble in ether reacts in ethanol |
| Thermochemistry | |
| 40.2 J/mol K | |
|
Std molar
entropy (S |
30 K/mol K |
|
Std enthalpy of
formation (ΔfH |
-11.4 kJ/mol |
|
Gibbs free energy (ΔfG˚)
|
46.4 kJ/mol |
| Related compounds | |
|
Related compounds
|
Lithium aluminium hydride, diborane |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Aluminic hydride
Aluminium(III) hydride
Aluminium trihydride
Aluminium hydride (also known as alane or alumane) is an inorganic compound with the formula AlH3. It is a colourless pyrophoric solid. Although rarely encountered outside of research laboratories, alane and its derivatives are used as reducing agents in organic synthesis.
Alane is a polymer. Hence, its formula is sometimes represented with the formula (AlH3)n. Alane forms numerous polymorphs, which are named α-alane, α’-alane, β-alane, γ-alane, δ-alane, ε-alane and ζ-alane. α-Alane has a cubic or rhombohedral morphology, whereas α’-alane forms needle-like crystals and γ-alane forms a bundle of fused needles. Alane is soluble in THF and ether. The rate of the precipitation of solid alane from ether varies with the preparation method.
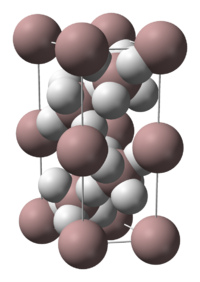
The crystal structure of α-alane has been determined and features aluminium atoms surrounded by 6 hydrogen atoms that bridge to 6 other aluminium atoms. The Al-H distances are all equivalent (172pm) and the Al-H-Al angle is 141°.
α-Alane is the most thermally stable polymorph. β-alane and γ-alane are produced together, and convert to α-alane upon heating. δ, ε, and θ-alane are produced in still other crystallization conditions. Although they are less thermally stable, δ, ε, and θ polymorphs do not convert into α-alane upon heating.
Monomeric AlH3 has been isolated at low temperature in a solid noble gas matrix and shown to be planar. The dimer Al2H6 has been isolated in solid hydrogen. It is isostructural with diborane (B2H6) and digallane (Ga2H6).
Aluminium hydrides and various complexes thereof have long been known. Its first synthesis published in 1947, and a patent for the synthesis was assigned in 1999. Aluminium hydride is prepared by treating lithium aluminium hydride with aluminium trichloride. The procedure is intricate, attention must be given to the removal of lithium chloride.
...
Wikipedia
