Adrenochrome
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
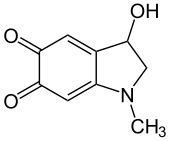
3-Hydroxy-1-methyl-2,3-dihydro-1H-indole-5,6-dione
|
|
| Other names
Adraxone; Pink adrenaline
|
|
| Identifiers | |
|
54-06-8 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
5687 |
| ECHA InfoCard | 100.000.176 |
| PubChem | 5898 |
|
|
|
|
| Properties | |
| C9H9NO3 | |
| Molar mass | 179.18 g·mol−1 |
| Density | 3.264 g/cm³ |
| Boiling point | (decomposes, 115-120 °C) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Adrenochrome is a chemical compound with the molecular formula C9H9NO3 produced by the oxidation of adrenaline (epinephrine). The derivative carbazochrome is a hemostatic medication. Despite a similarity in chemical names, it is unrelated to chrome or chromium.
In vivo, adrenochrome is synthesized by the oxidation of epinephrine. In vitro, silver oxide (Ag2O) is used as an oxidizing agent. Its presence is detected in solution by a pink color. The color turns brown upon polymerization.
Several small-scale studies (involving 15 or fewer test subjects) conducted in the 1950s and 1960s reported that adrenochrome triggered psychotic reactions such as thought disorder, derealization, and euphoria. Researchers Abram Hoffer and Humphry Osmond claimed that adrenochrome is a neurotoxic, psychotomimetic substance and may play a role in schizophrenia and other mental illnesses. In what they called the "adrenochrome hypothesis", they speculated that megadoses of vitamin C and niacin could cure schizophrenia by reducing brain adrenochrome. However, these hypotheses have never been scientifically accepted; adrenochrome is not currently believed to have any psychedelic properties.
...
Wikipedia
