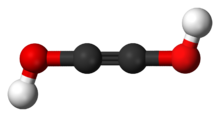
Acetylenediol
 |
|
| Names | |
|---|---|
| Other names
ethynediol, dihydroxyacetylene
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C2H2O2 | |
| Molar mass | 58,07 g/mol |
| Boiling point | dec. |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Acetylenediol, or ethynediol, is a chemical substance with formula HO-C≡C-OH. It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal H(C=O)2H is well known.
Acetylenediol was first observed in the gas-phase by mass spectrometry. The compound was later obtained by photolysis of squaric acid in a solid argon matrix at 10 K (−263.1 °C).
Although the diol has only fleeting existence in concentrated form, salts of the acetylenediolate (ethynediolate) dianion (O-C≡C-O)2− are well known. These organometallic compounds (specifically, alkoxides) are formally derived from ethynediol by loss of two hydrogen ions, but they are not normally generated in that way.
The typical synthesis route for these salts is the reduction of carbon monoxide. Potassium acetylenediolate (K2C2O2) was first obtained by Liebig in 1834, from the reaction of carbon monoxide with metallic potassium; but for a long time the product was assumed to be "potassium carbonyl" (KCO). Over the next 130 years were described the "carbonyls" of sodium (Johannis, 1893), barium (Gunz and Mentrel, 1903), strontium (Roederer, 1906), and lithium, rubidium, and caesium (Pearson, 1933). The reaction was eventually shown to yield a mixture of the potassium acetylenediolate K
2C
2O
2 and potassium benzenehexolate K
6C
6O
6.
...
Wikipedia
