5-API
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.236.959 |
| Chemical and physical data | |
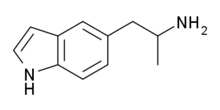
| Formula | C11H14N2 |
| Molar mass | 174.24 g/mol |
| 3D model (Jmol) | |
|
|
|
|
5-(2-Aminopropyl)indole (5-API, 5-IT, PAL-571) is an indole and phenethylamine derivative with empathogenic effects. Its preparation was first reported by Albert Hofmann in 1962. It is a designer drug that has been openly sold as a recreational drug by online vendors since 2011.
Although 5-IT is a positional isomer of the tryptamine drug αMT, the compound is not itself a tryptamine as the indole ring is substituted at the 5 position rather than at the 3 position. The compound is closer chemically to phenethylamine derivatives such as 5-APB. This is reflected in the compound's effects when used as a drug, which are reportedly stimulating rather than psychedelic.
5-IT acts as a triple monoamine releasing agent with EC50 values of 12.9 nM for dopamine, 13.3 nM for norepinephrine and 104.8 nM for serotonin and also as MAO-A inhibitor.
Alexander Shulgin wrote briefly about 5-IT in TiHKAL saying: "at 20 milligrams orally, [it] is a long-lived stimulant producing increased heart-rate, anorexia, diuresis, and slight hyperthermia for about twelve hours." As 5-IT is not a tryptamine and thus not within the scope of the book, it is not discussed in any more detail than this.
The following symptoms can indicate 5-IT has been ingested: hyperthermia, tachycardia, increased blood pressure, dilated pupils (mydriasis), agitation, excessive sweating, jaw clenching, insomnia, disorientation, restlessness, anxiety, and tremor. It is an MAOI, and when combined with a contraindicated substance, it can lead to death.
...
Wikipedia
