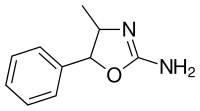
4-methylaminorex
 |
|
 |
|
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration |
Oral, Vaporized, , Injected |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 62% oral; 79% nasal; 91 - 93.5% smoked; 100% IV |
| Metabolism | Hepatic |
| Biological half-life | 10-19 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number |
3568-94-3 29493-77-4 - (±)-cis isomers |
| PubChem (CID) | 92196 |
| DrugBank |
DB01447 |
| ChemSpider |
83237 |
| Chemical and physical data | |
| Formula | C10H12N2O |
| Molar mass | 176.21 |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
|
|
|
|
|
|
|
4-Methylaminorex (4-MAR, 4-MAX) is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street names "U4Euh" ("Euphoria") and "Ice". It is banned in many countries as a stimulant.
4-Methylaminorex has effects comparable to methamphetamine but with a longer duration.
The results of animal experiments conducted with this drug suggest that it has an abuse liability similar to cocaine and amphetamine. One study found that, "stimulus properties of racemic cis, racemic trans, and all four individual optical isomers of 4-methylaminorex were examined in rats trained to discriminate 1 mg/kg of S(+)amphetamine sulfate from saline. The S(+)amphetamine stimulus generalized to all of the agents investigated". A second study in which rats trained to discriminate either 0.75 mg/kg S(+)-amphetamine or 1.5 mg/kg fenfluramine from saline generalized to aminorex as amphetamine stimulus but not to fenfluramine. Rats trained to discriminate 8 mg/kg cocaine from saline generalized 4-methylaminorex to cocaine-stimulus. The reinforcing effects of cis-4-methylaminorex were determined in two models of intravenous drug self-administration in primates. Vehicle or 4-methylaminorex doses were substituted for cocaine. One of the two different doses of 4-methylaminorex maintained self-administration behavior above vehicle control levels.
4-Methylaminorex exists as four stereoisomers - (±)-cis and (±)-trans. The (±)-cis isomers are the form used recreationally. The (±)-cis isomers [racemate (1:1-mixture) of the (4R,5S)-isomer and the enantiomeric (4S,5R)-isomer] generally synthesized from dl-phenylpropanolamine in one step by cyclization with cyanogen bromide (sometimes prepared in situ by reacting sodium cyanide with bromine). Alternate synthesis routes generally involve more steps, such as replacing cyanogen bromide with sodium or potassium cyanate to form an intermediate and then reacting it with concentrated hydrochloric acid. A method reported in microgram replaced the need for a separate addition of hydrochloric acid by starting with the hydrochloride salt of the dl-phenylpropanolamine but side-products are noted. The (±)-trans isomers [racemate (1:1-mixture) of the (4S,5S)-isomer and the enantiomeric (4R,5R)-isomer] are synthesized in the same manner above but dl-norephedrine is used as the starting material instead. The cyanate reaction proceeds differently from the cyanogen bromide and transforms norephedrine into trans-4-methylaminorex instead, as noted in the DEA micrograph. The cyanogen bromide, by comparison, transformed norephedrine into the cis isomer and norpseudoephedrine into the trans isomers of the final product.
...
Wikipedia
