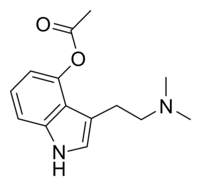
4-AcO-DMT
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral, IV, intranasal, rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| Synonyms | 4-Acetoxy-N,N-dimethyltryptamine, 3-(2'-dimethylaminoethyl)-4-acetoxy-indole |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C14H18N2O2 |
| Molar mass | 246.3049 g/mol |
| 3D model (Jmol) | |
| Melting point | 172 to 173 °C (342 to 343 °F) |
|
|
|
|
O-Acetylpsilocin (also known as psilacetin, 4-acetoxy-DMT, or 4-AcO-DMT) is a synthetically produced psychoactive drug and has been suggested by David Nichols to be a potentially useful alternative to psilocybin for pharmacological studies, as they are both believed to be prodrugs of psilocin. However, some users report that O-acetylpsilocin's subjective effects differ from that of psilocybin and psilocin. It is the acetylated form of the psilocybin mushroom alkaloid psilocin and is a lower homolog of 4-AcO-MET, 4-AcO-DET, 4-AcO-MiPT and 4-AcO-DiPT.
O-Acetylpsilocin (psilacetin) and several other esters of psilocin were patented on January 16, 1963 by Sandoz Ltd. via Albert Hofmann & Franz Troxler. Despite this, psilacetin remains a psychedelic with a limited history of use. It is theorized to be a prodrug for psilocin, as is psilocybin, which is naturally occurring in various mushrooms. This is because the aromatic acetyl moiety on the 4th position is subject to deacetylation by acidic conditions such as those found in the stomach. Psilacetin is O-acetylated psilocin, whereas psilocybin is O-phosphorylated.
O-Acetylpsilocin can be obtained by acetylation of psilocin under alkaline or strongly acidic conditions. It is a synthetic compound. However, it is believed to be a prodrug of psilocin, which is natural and occurs in many mushrooms along with psilocybin. O-Acetylpsilocin is more resistant than psilocin to oxidation under basic conditions due to its acetoxy group. While O-acetylpsilocin is not well researched (considered by many to be a research chemical, as opposed to psilocin and psilocybin), it is not as difficult to produce as psilocybin, and may be an appropriate substitute for psilocybin in psychedelic research because of their similar mechanism of action.
...
Wikipedia
