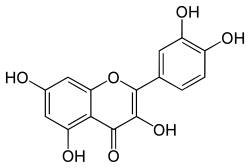
3,5,7,3',4'-pentahydroxyflavone
 |
|
 |
|
| Names | |
|---|---|
| Pronunciation | /ˈkwɜːrsɪtɪn/ |
|
IUPAC name
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one
|
|
| Other names
5,7,3′,4′-flavon-3-ol, Sophoretin, Meletin, Quercetine, Xanthaurine, Quercetol, Quercitin, Quertine, Flavin meletin
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.807 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C15H10O7 | |
| Molar mass | 302.236 g/mol |
| Appearance | yellow crystalline powder |
| Density | 1.799 g/cm3 |
| Melting point | 316 °C (601 °F; 589 K) |
| Practically insoluble in water; soluble in aqueous alkaline solutions | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Quercetin is a plant polyphenol from the flavonoid group, found in many fruits, vegetables, leaves, and grains. It can be used as an ingredient in supplements, beverages, or foods.
Quercetin is a flavonoid widely distributed in nature. The name has been used since 1857, and is derived from quercetum (oak forest), after Quercus. It is a naturally occurring polar auxin transport inhibitor.
Quercetin is one of the most abundant dietary flavonoids with an average daily consumption of 25–50 mgs.
In red onions, higher concentrations of quercetin occur in the outermost rings and in the part closest to the root, the latter being the part of the plant with the highest concentration. One study found that organically grown tomatoes had 79% more quercetin than non-organically grown fruit. Quercetin is present in various kinds of honey from different plant sources.
In plants, phenylalanine is converted to 4-coumaroyl-CoA in a series of steps known as the general phenylpropanoid pathway using phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA-ligase. One molecule of 4-coumaroyl-CoA is added to three molecules of malonyl-CoA to form tetrahydroxychalcone using 7,2′-dihydroxy-4′-methoxyisoflavanol synthase. Tetrahydroxychalcone is then converted into naringenin using chalcone isomerase.
Naringenin is converted into eriodictyol using flavanoid 3′-hydroxylase. Eriodictyol is then converted into dihydroquercetin with flavanone 3-hydroxylase, which is then converted into quercetin using flavonol synthase.
...
Wikipedia
