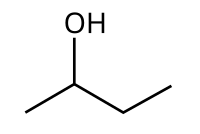
2-Butanol
 |
|
| Names | |
|---|---|
|
IUPAC name
Butan-2-ol
|
|
| Other names
sec-Butanol
sec-Butyl alcohol, 2-Butanol 2-Butyl alcohol |
|
| Identifiers | |
|
78-92-2 14898-79-4 (R) 4221-99-2 (S) |
|
| 3D model (Jmol) | Interactive image |
| 773649 1718764 (R) |
|
| ChEBI |
CHEBI:35687 |
| ChEMBL |
ChEMBL45462 |
| ChemSpider |
6320 76392 (R) 392543 (S) |
| DrugBank |
DB02606 |
| ECHA InfoCard | 100.001.053 |
| EC Number | 201-158-5 |
| 1686 396584 (R) |
|
| MeSH | 2-butanol |
| PubChem |
6568 84682 (R) 444683 (S) |
| RTECS number | EO1750000 |
| UN number | 1120 |
|
|
|
|
| Properties | |
| C4H10O | |
| Molar mass | 74.12 g·mol−1 |
| Density | 0.808 g cm−3 |
| Melting point | −115 °C; −175 °F; 158 K |
| Boiling point | 98 to 100 °C; 208 to 212 °F; 371 to 373 K |
| 290 g/L | |
| log P | 0.683 |
| Vapor pressure | 1.67 kPa (at 20 °C) |
| -57.683·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.3978 (at 20 °C) |
| Thermochemistry | |
| 197.1 J K−1 mol−1 | |
|
Std molar
entropy (S |
213.1 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−343.3–−342.1 kJ mol−1 |
|
Std enthalpy of
combustion (ΔcH |
−2.6611–−2.6601 MJ mol−1 |
| Hazards | |
| Safety data sheet | inchem.org |
| GHS pictograms |
 
|
| GHS signal word | WARNING |
| H226, H319, H335, H336 | |
| P261, P305+351+338 | |
|
EU classification (DSD)
|
|
| R-phrases | R10, R36/37, R67 |
| S-phrases | (S2), S7/9, S13, S24/25, S26, S46 |
| NFPA 704 | |
| Flash point | 22 to 27 °C (72 to 81 °F; 295 to 300 K) |
| 405 °C (761 °F; 678 K) | |
| Explosive limits | 1.7–9.8% |
| Lethal dose or concentration (LD, LC): | |
|
LCLo (lowest published)
|
16,000 ppm (rat, 4 hr) 10,670 ppm (mouse, 3.75 hr) 16,000 ppm (mouse, 2.67 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 150 ppm (450 mg/m3) |
|
REL (Recommended)
|
TWA 100 ppm (305 mg/m3) ST 150 ppm (455 mg/m3) |
|
IDLH (Immediate danger)
|
2000 ppm |
| Related compounds | |
|
Related butanols
|
n-Butanol Isobutanol tert-Butanol |
|
Related compounds
|
Butanone |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
sec-Butyl alcohol, 2-Butanol
1718764 (R)
1718763 (S)
396584 (R)
25655 (S)
2-Butanol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. This secondary alcohol is a flammable, colorless liquid that is soluble in 3 parts water and completely miscible with polar organic solvents such as ethers and other alcohols. It is produced on a large scale, primarily as a precursor to the industrial solvent methyl ethyl ketone. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-butanol and (S)-(+)-2-butanol. It is normally found as an equal mixture of the two stereoisomers — a racemic mixture.
2-Butanol is manufactured industrially by the hydration of 1-butene or 2-butene:
Sulfuric acid is used as a catalyst for this conversion.
In the lab it can be prepared via Grignard reaction by reacting ethylmagnesium bromide with acetaldehyde in dried diethyl ether or tetrahydrofuran.
Although some 2-butanol is used as a solvent, it is mainly converted to butanone ("MEK"), which is an important industrial solvent and found in many domestic cleaning agents and paint removers. Volatile esters of 2-butanol have pleasant aromas and are used in small amounts as perfumes or in artificial flavors.
...
Wikipedia

