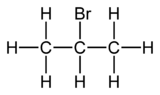
2-Bromopropane
 |
|||
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
2-Bromopropane
|
|||
| Other names
Isopropyl bromide
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| 741852 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.778 | ||
| EC Number | 200-855-1 | ||
| MeSH | 2-bromopropane | ||
|
PubChem CID
|
|||
| RTECS number | TX4111000 | ||
| UN number | 2344 | ||
|
|||
|
|||
| Properties | |||
| C3H7Br | |||
| Molar mass | 122.99 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.31 g mL−1 | ||
| Melting point | −89.0 °C; −128.1 °F; 184.2 K | ||
| Boiling point | 59 to 61 °C; 138 to 142 °F; 332 to 334 K | ||
| 3.2 g L−1 (at 20 °C) | |||
| log P | 2.136 | ||
| Vapor pressure | 32 kPa (at 20 °C) | ||
|
Henry's law
constant (kH) |
1.0 μmol Pa−1 mol−1 | ||
|
Refractive index (nD)
|
1.4251 | ||
| Viscosity | 4.894 mPa s (at 20 °C) | ||
| Thermochemistry | |||
| 135.6 J K mol−1 | |||
|
Std enthalpy of
formation (ΔfH |
−129 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−2.0537–−2.0501 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |
 
|
||
| GHS signal word | DANGER | ||
| H225, H360, H373 | |||
| P210, P308+313 | |||
| NFPA 704 | |||
| Flash point | 19 °C (66 °F; 292 K) | ||
| Related compounds | |||
|
Related alkanes
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2-Bromopropane, also known as isopropyl bromide and 2-propyl bromide, is the halogenated hydrocarbon with the formula CH3CHBrCH3. It is a colorless liquid. It is used for introducing the isopropyl functional group in organic synthesis. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid.
2-Bromopropane is commercially available. It may be prepared in the ordinary manner of alkyl bromides, by reacting isopropanol with phosphorus and bromine, or with phosphorus tribromide.
The bromine atom is at the secondary position, which allows the molecule to undergo dehydrohalogenation easily to give propene, which escapes as a gas. Consequently, this reagent is used in conjunction with mild bases, such as potassium carbonate, rather than strong ones.
Alkylating agents are often carcinogenic.
...
Wikipedia



