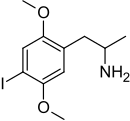
2,5-Dimethoxy-4-iodoamphetamine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
1-(4-Iodo-2,5-dimethoxyphenyl)propan-2-amine
|
|
| Identifiers | |
|
|
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.164.735 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C11H16INO2 | |
| Molar mass | 321.1558 g/mol |
| Melting point | 201.5 °C (394.7 °F; 474.6 K) (hydrochloride) |
| 10 mg/mL | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
2,5-Dimethoxy-4-iodoamphetamine (DOI) is a psychedelic drug and a substituted amphetamine. Unlike other substituted amphetamines, however, it is not a stimulant. DOI has a stereocenter and R-(−)-DOI is the more active stereoisomer. In neuroscience research, [125I]-R-(−)-DOI is used as a radioligand and indicator of the presence of 5-HT2A serotonin receptors. DOI's effects have been compared to LSD, although there are differences that experienced users can distinguish. Besides the longer duration, the trip tends to be more energetic than an LSD trip, with more body load and a different subjective visual experience. The after effects include residual stimulation and difficulty sleeping, which, depending on the dose, may persist for days. It is sometimes sold as a substitute for LSD, or even sold falsely as LSD, which may be dangerous because DOI does not have the same established safety profile as LSD.
Research suggests that administration of (R)-DOI blocks pulmonary inflammation, mucus hyper-production, airway hyper-responsiveness and turns off key genes in in-lung immune response. These effects block the development of allergic asthma in a mouse model.
DOI is a 5-HT2A, 5-HT2B and 5-HT2C receptor agonist.
DOI has been shown to be an extremely potent inhibitor of tumour necrosis factor-alpha inflammation at picomolar concentrations in cell studies. TNF-alpha is an important target for research into degenerative conditions such as rheumatoid arthritis and Alzheimer's disease, where the disease process involves tissue damage through chronic inflammation. This could make DOI and other 5-HT2A agonists an entirely new area for development of novel treatments for these conditions.
...
Wikipedia
