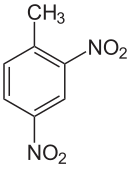
2,4-Dinitrotoluene
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
1-methyl-2,4-dinitro benzene
|
|
| Other names
Dinitrotoluol, Methyldinitrobenzene
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.046 |
| KEGG | |
|
PubChem CID
|
|
| UN number |
Molten: 1600 Solid or liquid: 2038 |
|
|
|
|
| Properties | |
| C7H6N2O4 | |
| Molar mass | 182.134 g/mol |
| Appearance | Pale yellow to orange crystalline solid |
| Density | 1.52 g/cm3 |
| Melting point | 70 °C (158 °F; 343 K) |
| Boiling point | Decomposes at 250–300 °C |
| Vapor pressure | 1.47X10-4 mm Hg @ 22 C |
| Hazards | |
| Main hazards | carcinogen, combustible (though difficult to ignite) |
| Flash point | 207 °C; 404 °F; 480 K |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
216 mg/kg (oral, rat, 3,5-isomer) 1,954 mg/kg (oral, mouse, 2,4-isomer) |
|
LDLo (lowest published)
|
27 mg/kg (2,4 isomer, cat, oral) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 1.5 mg/m3 [skin] |
|
REL (Recommended)
|
Ca TWA 1.5 mg/m3 [skin] |
|
IDLH (Immediate danger)
|
Ca [50 mg/m3] |
| Explosive data | |
| Shock sensitivity | Insensitive |
| Friction sensitivity | Very low |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
2,4-Dinitrotoluene (DNT) or dinitro is an organic compound with the formula C7H6N2O4. This pale yellow crystalline solid is well known as a precursor to trinitrotoluene (TNT) but is mainly produced as a precursor to toluene diisocyanate.
Six isomers are possible for dinitrotoluene. The most common one is 2,4-dinitrotoluene. The nitration of toluene gives sequentially mononitrotoluene, DNT, and finally TNT. 2,4-DNT is the principal product from dinitration, the other main product being about 30% 2,6-DNT. The nitration of 4-nitrotoluene gives 2,4-DNT.
Most DNT is used in the production of toluene diisocyanate, which is used to produce flexible polyurethane foams. DNT is hydrogenated to produce 2,4-toluenediamine, which in turn is phosgenated to give toluene diisocyanate. In this way, about 1.4 billion kilograms are produced annually, as of the years 1999–2000. Other uses include the explosives industry. It is not used by itself as an explosive, but some of the production is converted to TNT.
Dinitrotoluene is frequently used as a plasticizer, deterrent coating, and burn rate modifier in propellants (e.g., smokeless gunpowders). As it is carcinogenic and toxic, modern formulations tend to avoid its use. In this application it is often used together with dibutyl phthalate.
...
Wikipedia
