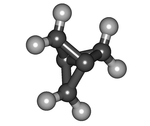
1.1.1-Propellane
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Tricyclo[1.1.1.01,3]pentane
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C5H6 | |||
| Molar mass | 66.10 g·mol−1 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
[1.1.1]Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(>CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.
[1.1.1]Propellane is a highly strained molecule. The bonds of the two central carbon atoms have an inverted tetrahedral geometry, and the length of the central bond is 160 pm. The strength of that bond is disputed; estimates vary from 59–65 kcal/mol to no strength at all. The energy of the biradical state (without the central bond and unfilled central carbons) is calculated to be 80 kcal/mol higher. The compound is highly unstable, and at 114 °C it will spontaneously isomerize to 3-methylidenecyclobutene with a half-life of 5 minutes. Its strain energy is estimated to be 102 kcal/mol (427 kJ/mol).
The type of bonding in this molecule has been explained in terms of charge-shift bonding.
[1.1.1]Propellane was first synthesized by K. Wiberg and F. Walker in 1982, according to the following scheme:
Synthesis begins with conversion of the 1,3-di-carboxylic acid of bicyclo[1.1.1]pentane 1 in a Hunsdiecker reaction to the corresponding dibromide 2 followed by a coupling reaction with n-butyllithium. The final product 3 was isolated by column chromatography at −30 °C.
...
Wikipedia