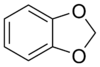
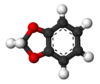
1,3-Benzodioxole
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2H-1,3-Benzodioxole
|
|||
| Other names
1,3-Benzodioxole
Benzo[d][1,3]dioxole 1,2-[Methylenebis(oxy)]benzene 1,2-Methylenedioxybenzene (no longer recommended) |
|||
| Identifiers | |||
|
274-09-9 |
|||
| 3D model (Jmol) |
Interactive image Interactive image |
||
| 115506 | |||
| ChEBI |
CHEBI:38732 |
||
| ChemSpider |
13881169 |
||
| ECHA InfoCard | 100.005.448 | ||
| EC Number | 205-992-0 | ||
| MeSH | 1,3-Benzodioxole | ||
| PubChem | 9229 | ||
| RTECS number | DA5600000 | ||
| UN number | 1993 | ||
|
|||
|
|||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.12 g·mol−1 | ||
| Density | 1.064 g cm−3 | ||
| Boiling point | 172–173 °C (342–343 °F; 445–446 K) | ||
| log P | 2.08 | ||
| Vapor pressure | 1.6 kPa | ||
| Thermochemistry | |||
|
Std enthalpy of
combustion (ΔcH |
-3.428 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  |
||
| GHS signal word | WARNING | ||
| H302, H332 | |||
|
EU classification (DSD)
|
|||
| R-phrases | R20/22 | ||
| S-phrases | S22, S24/25 | ||
| NFPA 704 | |||
| Flash point | 61 °C (142 °F; 334 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
1,3-Benzodioxole (1,2-methylenedioxybenzene) is an organic compound with the formula C6H4O2CH2. The compound is classified as benzene derivative and a heterocyclic compound containing the methylenedioxy functional group. It is a colorless liquid.
Although benzodioxozole is not particularly important, many related compounds containing the methylenedioxyphenyl group are bioactive, and thus are found in pesticides and pharmaceuticals.
1,3-Benzodioxole can be synthesized from catechol with disubstituted halomethanes.
...
Wikipedia