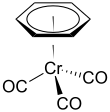
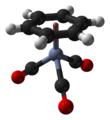
(Benzene)chromium tricarbonyl
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
(benzene)tricarbonylchromium
|
|||
| Other names
benzene tricarbonyl chromium, (benzene)chromium tricarbonyl, Benchrotrene, pi-benzenetricarbonylchromium
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.031.939 | ||
| EC Number | 235-146-6 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| Cr(C6H6)(CO)3 | |||
| Molar mass | 214.14 g/mol | ||
| Appearance | solid yellow crystals | ||
| Melting point | 163 to 166 °C (325 to 331 °F; 436 to 439 K) | ||
| nonsoluble | |||
| Solubility | THF, ether, benzene | ||
| Structure | |||
| tetrahedral, "piano stool" | |||
| Hazards | |||
| Main hazards | Harmful through inhalation, contact with skin, or swallowed | ||
| GHS pictograms |  |
||
| GHS signal word | Warning | ||
| H302, H312, H332 | |||
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
(Benzene)chromium tricarbonyl is an organometallic compound with the formula Cr(C6H6)(CO)3. This yellow crystalline solid compound is soluble in common nonpolar organic solvents. The molecule adopts a geometry known as “piano stool” because of the planar arrangement of the aryl group and the presence of three CO ligands as "legs" on the chromium-bond axis.
(Benzene)tricarbonylchromium was first reported in 1957 by Fischer and Öfele, who prepared the compound by the carbonylation of bis(benzene)chromium. They obtained mainly chromium carbonyl (Cr(CO)6) and traces of Cr(C6H6)(CO)3. The synthesis was optimized through the reaction of Cr(CO)6 and Cr(C6H6)2. For commercial purposes, a reaction of Cr(CO)6 and benzene is used:
The aromatic ring of (benzene)tricarbonylchromium is substantially more electrophilic than benzene itself, allowing it to undergo nucleophilic addition reactions.
It is also more acidic, undergoing lithiation upon treatment with n-butyllithium. The resulting organolithium compound can then be used as a nucleophile in various reactions, for example, with trimethylsilyl chloride:
(Benzene)tricarbonylchromium is a useful catalyst for the hydrogenation of 1,3-dienes. The product alkene results from 1,4-addition of hydrogen. The complex does not hydrogenate isolated double bonds.
...
Wikipedia