Β-glucuronidase
| beta-glucuronidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|

Glucuronidase Homotetramer
(assumed biological unit) |
|||||||||
| Identifiers | |||||||||
| EC number | 3.2.1.31 | ||||||||
| CAS number | 9001-45-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
| glucuronidase, beta | |
|---|---|
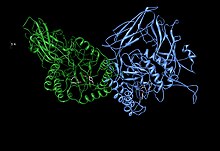
Beta-glucuronidase asymmetric unit showing active site residues Glu451, Tyr504, and Glu540, along with the potentially supporting Asn450 residue
|
|
| Identifiers | |
| Symbol | GUSB |
| Entrez | 2990 |
| HUGO | 4696 |
| OMIM | 611499 |
| RefSeq | NM_000181 |
| UniProt | P08236 |
| Other data | |
| EC number | 3.2.1.31 |
| Locus | Chr. 7 q11.21 |
Beta-glucuronidases are members of the glycosidase family of enzymes that catalyze breakdown of complex carbohydrates. Human β-glucuronidase is a type of glucuronidase (a member of glycosidase Family 2) that catalyzes hydrolysis of β-D-glucuronic acid residues from the non-reducing end of mucopolysaccharides (also referred to as glycosaminoglycans) such as heparan sulfate. Human β-glucuronidase is located in the lysosome. In the gut, brush border β-glucuronidase converts conjugated bilirubin to the unconjugated form for reabsorption. Beta-glucuronidase is also present in breast milk, which contributes to neonatal jaundice. The protein is encoded by the GUSB gene.
Human β-glucuronidase is synthesized as an 80 kDa monomer (653 amino acids) before proteolysis removes 18 amino acids from the C-terminal end to form a 78 kDa monomer. Beta-glucuronidase exists as a 332 kDa homotetramer. Beta-glucuronidase contains several notable structural formations, including a type of beta barrel known as a jelly roll barrel and a TIM barrel.
Human β-glucuronidase is homologous to the Escherichia coli enzyme β-galactosidase. This homologous relationship, along with the knowledge that glycosidases often perform hydrolysis catalyzed by two acidic residues, enabled the development of a mechanistic hypothesis. This hypothesis proposes that the two glutamic acid residues Glu540 and Glu451 are the nucleophilic and acidic residues, respectively, and that the tyrosine residue Tyr504 is also involved in catalysis. In support of this hypothesis, experimental mutations in any of these three residues result in large decreases of enzymatic activity. Increased activity of an E451A mutant enzyme (where Glu451 is replaced with an alanine residue) after addition of azide is consistent with Glu451 as the acid/base residue. Using analysis of labeled β-glucuronidase peptides after hydrolysis of a substrate that enters a very stable intermediate stage, researchers have determined that Glu540 is the nucleophilic residue.
...
Wikipedia
