Zoledronate
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | M05BA08 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 22% |
| Metabolism | Nil |
| Biological half-life | 146 hours |
| Excretion | Renal (partial) |
| Identifiers | |
|
|
| CAS Number | 118072-93-8 |
| PubChem (CID) | 68740 |
| IUPHAR/BPS | 3177 |
| DrugBank | DB00399 |
| ChemSpider | 61986 |
| UNII |
70HZ18PH24 |
| KEGG |
D08689 |
| ChEMBL |
CHEMBL924 |
| PDB ligand ID | ZOL (PDBe, RCSB PDB) |
| Chemical and physical data | |
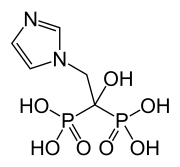
| Formula | C5H10N2O7P2 |
| Molar mass | 272.09 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Zoledronic acid (INN) or zoledronate is a bisphosphonate drug given intravenously to treat some bone diseases. It is sold under many trade names worldwide.
Zoledronic acid slows down bone resorption, allowing the bone-forming cells time to rebuild normal bone and allowing bone remodeling.
Zometa is used to prevent skeletal fractures in patients with cancers such as multiple myeloma and prostate cancer, as well as for treating osteoporosis. It can also be used to treat hypercalcemia of malignancy and can be helpful for treating pain from bone metastases.
It can be administered at home rather than in hospital. Such administration has shown safety and quality-of-life benefits in breast cancer patients with bone metastases.
Marketed as Aclasta (in Australia) or Reclast (in the US), zoledronic acid may be given as a 5 mg infusion once per year for treatment of osteoporosis in men and post-menopausal women at increased risk of fracture.
In 2007, the U.S. Food and Drug Administration (FDA) also approved Reclast for the treatment of postmenopausal osteoporosis.
As Reclast a single dose of 5 mg is used for the treatment of Paget's disease.
Side-effects can include fatigue, anemia, muscle aches, fever, and/or swelling in the feet or legs. Flu-like symptoms are commonly experienced after the first zoledronate infusion, although not subsequent infusions, and are thought to occur because of its potential to activate human γδ T cells (gamma/delta T cells).
...
Wikipedia
