Williamson synthesis
| Williamson ether synthesis | |
|---|---|
| Named after | Alexander William Williamson |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | williamson-synthesis |
| RSC ontology ID | RXNO:0000090 |
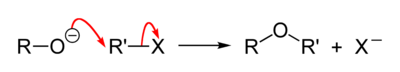
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers.
The general reaction mechanism is as follows:
An example is the reaction of sodium ethoxide with chloroethane to form diethyl ether and sodium chloride:
The Williamson reaction is of broad scope, is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The intramolecular reaction of halohydrins in particular, gives epoxides.
In the case of asymmetrical ethers there are two possibilities for the choice of reactants, and one is usually preferable either on the basis of availability or reactivity. The Williamson reaction is also frequently used to prepare an ether indirectly from two alcohols. One of the alcohols is first converted to a leaving group (usually tosylate), then the two are reacted together.
...
Wikipedia