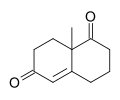
Wieland-Miescher ketone
 |
|
| Names | |
|---|---|
|
IUPAC name
8a-Methyl-3,4,7,8-tetrahydro-2H-naphthalene-1,6-dione
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.039.497 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C11H14O2 | |
| Molar mass | 178.23 g/mol |
| Melting point | 47 to 50 °C (117 to 122 °F; 320 to 323 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
The Wieland–Miescher ketone is a racemic bicyclic diketone (enedione) and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer, antimicrobial, antiviral, antineurodegenerative and immunomodulatory activities. The reagent is named after two chemists from Ciba Geigy, Karl Miescher and Peter Wieland (not to be confused with Heinrich Otto Wieland). Examples of syntheses performed using the optically active enantiomer of this diketone as a starting material are that of ancistrofuran and the Danishefsky total synthesis of Taxol.
Most advances in total synthesis methods starting from Wieland–Miescher ketone were fueled by the search for alternative methods for the industrial synthesis of contraceptive and other medicinally relevant steroids, an area of research that flourished in the 1960s and 1970s. Wieland–Miescher ketone contains the AB-ring structure of steroids and is for this reason an attractive starting material for the steroid skeleton, an approach used in one successful synthesis of adrenosterone.
The original Wieland–Miescher ketone is racemic and prepared in a Robinson annulation of 2-methyl-1,3-cyclohexanedione and methyl vinyl ketone. The intermediate alcohol is not isolated. The required 2-methyl-1,3-cyclohexanedione can be prepared from resorcinol by hydrogenation over Raney nickel to dihydroresorcinol as the enolate followed by alkylation with methyl iodide.
...
Wikipedia
