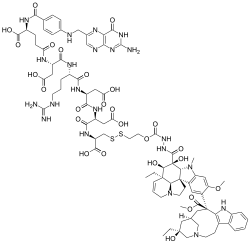
Vintafolide
 |
|
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| Synonyms | EC-145 |
| CAS Number | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.234.085 |
| Chemical and physical data | |
| Formula | C86H109N21O26S2 |
| Molar mass | 1917 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Vintafolide is an investigational targeted cancer therapeutic currently under development by Endocyte and Merck & Co. It is a small molecule drug conjugate consisting of a small molecule targeting the folate receptor, which is overexpressed on certain cancers, such as ovarian cancer, and a potent chemotherapy drug, vinblastine.
Vintafolide is designed to deliver the toxic vinblastine drug selectively to cells expressing the folate receptor using folate targeting.
It is being developed with a companion imaging agent, etarfolatide, that identifies patients that express the folate receptor and thus would likely respond to the treatment with vintafolide.
A Phase 3 study evaluating vintafolide for the treatment of platinum-resistant ovarian cancer (PROCEED trial) and a Phase 2b study(TARGET trial) in non-small-cell lung carcinoma (NSCLC) are ongoing (in 2012).
A Marketing Authorization Application (MAA) filing for vintafolide and etarfolatide for the treatment of patients with folate receptor-positive platinum-resistant ovarian cancer in combination with doxorubicin, pegylated liposomal doxorubicin (PLD), has been accepted by the European Medicines Agency. The drug received orphan drug status in Europe in March 2012.Merck & Co. acquired the development and marketing rights to this experimental cancer drug from Endocyte in April 2012.Endocyte remains responsible for the development and commercialization of etarfolatide, a non-invasive companion imaging agent used to identify patients expressing the folate receptor that will likely respond to treatment with vintafolide.
...
Wikipedia
