Vinpocetine
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 56.6 +/- 8.9% |
| Metabolism | hepatic |
| Biological half-life | 2.54 +/- 0.48 hours |
| Excretion | renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.050.917 |
| Chemical and physical data | |
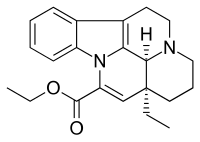
| Formula | C22H26N2O2 |
| Molar mass | 350.454 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Vinpocetine (brand names: Cavinton, Intelectol; chemical name: ethyl apovincaminate) is a synthetic derivative of the vinca alkaloid vincamine (sometimes described as "a synthetic ethyl ester of apovincamine"), an extract from the lesser periwinkle plant. Vinpocetine was first isolated from the plant in 1975 by the Hungarian chemist Csaba Szántay. The mass production of the synthetic compound was started in 1978 by the Hungarian pharmaceutical company Richter Gedeon.
Vinpocetine is not FDA approved in the United States for therapeutic use. The U.S. Food & Drug Administration (FDA) has ruled that vinpocetine, due to its synthetic nature and proposed therapeutic uses, was ineligible to be marketed as dietary supplement under the Federal Food, Drug, and Cosmetic Act (FDCA).
Vinpocetine has been reported to have cerebral blood-flow enhancing and neuroprotective effects, and has been used as a drug in Eastern Europe for the treatment of cerebrovascular disorders and age-related memory impairment.
As of 2003 only three controlled clinical trials had tested "older adults with memory problems". However, a 2003 Cochrane review determined that the results were inconclusive.
Kindling models in rats has shown vinpocetine to exhibit anticonvulsant properties. The most pronounced anticonvulsant effects were observed in Pentylenetetrazole (PTZ)-kindled rats although there was also an effect on amygdala-kindled and neocortically-kindled rats. Vinpocetine has also been shown to abolish [3H]Glu release after in vivo exposure to 4-aminopyridine (4-AP) which suggests an important mechanism for vinpocetine anticonvulsant activity.
...
Wikipedia
