Vaska's complex
 |
|
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
(SP-4-1)-carbonylchlorido
|
|
| Other names
Iridium(I)bis(triphenylphosphine)
carbonyl chloride Vaska's complex Vaska's compound |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.035.386 |
| EC Number | 238-941-6 |
|
|
|
|
| Properties | |
| IrCl(CO)[P(C6H5)3]2. | |
| Molar mass | 780.25 g/mol |
| Appearance | yellow crystals |
| Melting point | 215 °C (419 °F; 488 K) (decomposes) |
| Boiling point | 360 °C (680 °F; 633 K) |
| insol | |
| Structure | |
| sq. planar | |
| Hazards | |
| Main hazards | none |
| R-phrases | none |
| S-phrases | 22-24/25 |
| Related compounds | |
|
Other anions
|
IrI(CO)[P(C6H5)3]2 |
|
Other cations
|
RhCl(CO)[P(C6H5)3]2 |
|
Related compounds
|
Pd[P(C6H5)3]4 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
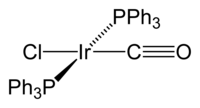
Vaska's complex is the trivial name for the chemical compound trans-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO)[P(C6H5)3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a chloride ion. The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability to bind to O2 reversibly. It is a bright yellow crystalline solid.
The synthesis involves heating virtually any iridium chloride salt with triphenylphosphine and a carbon monoxide source. The most popular method uses dimethylformamide (DMF) as a solvent, and sometimes aniline is added to accelerate the reaction. Another popular solvent is 2-methoxyethanol. The reaction is typically conducted under nitrogen. In the synthesis, triphenylphosphine serves as both a ligand and a reductant, and the carbonyl ligand is derived by decomposition of dimethylformamide, probably via a deinsertion of an intermediate Ir-C(O)H species. The following is a possible balanced equation for this complicated reaction.
Typical sources of iridium used in this preparation are IrCl3·xH2O and H2IrCl6.
...
Wikipedia
