Type IV collagen C4 domain
| C4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
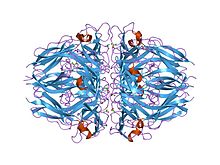
the 1.9-a crystal structure of the noncollagenous (nc1) domain of human placenta collagen iv shows stabilization via a novel type of covalent met-lys cross-link
|
|||||||||
| Identifiers | |||||||||
| Symbol | C4 | ||||||||
| Pfam | PF01413 | ||||||||
| Pfam clan | CL0056 | ||||||||
| InterPro | IPR001442 | ||||||||
| SMART | C4 | ||||||||
| PROSITE | PDOC00031 | ||||||||
| MEROPS | C47 | ||||||||
| SCOP | 1hra | ||||||||
| SUPERFAMILY | 1hra | ||||||||
| TCDB | 2.A.16 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
In molecular biology, the type IV collagen C4 domain (or collagen IV NC1 domain) is a duplicated domain present at the C-terminus of type IV collagens. Each type IV collagen contains a long triple-helical collagenous domain flanked by a short 7S domain of 25 amino acids and a globular non-collagenous C4 domain of ~230 amino acids at the N and C terminus, respectively. In protomer assembly, the C4 domains of three chains interact, forming an C4 trimer, to select and register chains for triple helix formation. In network assembly, the C4 trimers of two protomers interact, forming a C4 hexamer structure, to select and connect protomers.
The collagen IV C4 domain contains 12 cysteines, and all of them are involved in disulphide bonds. It folds into a tertiary structure with predominantly beta-strands. The collagen IV C4 domain is composed of two similarly folded subdomains stabilised by 3 intrachain dissulphide bonds involving the following pairs: C1-C6, C2-C5, and C3-C4. Each subdomain represents a compact disulphide-stabilised triangular structure, from which a finger-like hairpin loop projects into an incompletely formed six-stranded beta-sheet of an adjacent subdomain of the same or of an adjacent chain clamping the subdomains tightly together.
This article incorporates text from the public domain Pfam and InterPro IPR001442
...
Wikipedia
