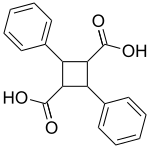
Truxillic acid
 |
|
| Names | |
|---|---|
|
IUPAC name
2,4-Diphenyl-1,3-cyclobutanedicarboxylic acid
|
|
| Identifiers | |
| 4462-95-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 70589 |
| ECHA InfoCard | 100.022.478 |
| PubChem | 78213 |
|
|
| Properties | |
| C18H16O4 | |
| Molar mass | 296.32 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Truxillic acids are any of several crystalline stereoisomeric cyclic dicarboxylic acids with the formula (C6H5)2C4H4(COOH)2 that yield cinnamic acid on distillation. They are obtained by a cycloaddition from cinnamic acid, where the two trans alkenes react head-to-tail. The isolated stereoisomers are called truxillic acids.
These compounds are found in a variety of plants, for example in coca.
These compounds have four chiral carbon atoms, which looks like there should be 16 (24) stereoisomers. However, the symmetry of the molecule allows for only five possibilities:
Incarvillateine, an alkaloid from the plant Incarvillea sinensis, is a derivative of α-truxillic acid.
...
Wikipedia
