Trospium chloride
 |
|
| Clinical data | |
|---|---|
| Trade names | Regurin, Sanctura, many generics |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
By mouth (tablets, capsules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 50–85% |
| Biological half-life | 20 hours |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.030.784 |
| Chemical and physical data | |
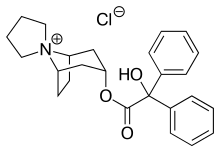
| Formula | C25H30ClNO3 |
| Molar mass | 427.964 g/mol |
| 3D model (JSmol) | |
|
|
|
|
Trospium chloride (INN) is used to treat overactive bladder.
It has side effects typical of drugs like it, namely dry mouth, stomach upset, and constipation; these side effects cause problems with people taking their medicine as directed. However it doesn't cause central nervous system side effects like other drugs of its class. It is pregnancy category C and is excreted somewhat in breast milk.
Chemically it is a quaternary ammonium cation which causes it to stay in periphery rather than crossing the blood-brain barrier. It works by causing the smooth muscle in the bladder to relax.
It was invented in the late 1960s in Germany and was first approved in Europe in 1999. It was first approved in the US in 2004, and an extended release version with once a day dosing was brought to market in 2007. It became generic in Europe in 2009 and in the US the first extended-release generic was approved in 2012.
Trospium chloride is used for the treatment of overactive bladder with symptoms of urge incontinence and frequent urination.
It shouldn't be used with people who retain urine, who have severe digestive conditions, myasthenia gravis, narrow-angle glaucoma, or tachyarrhythmia.
It should be used in caution with people who have problems with their autonomous nervous system (dysautonomia) or who have gastroesophageal reflux disease, or in whom fast heart rates are undesirable, such as people with hyperthyroidism, coronary artery disease and congestive heart failure.
...
Wikipedia
