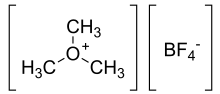
Trimethyloxonium tetrafluoroborate
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Trimethyloxonium tetrafluoroborate
|
|
| Other names
Trimethyloxonium fluoborate
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.360 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C3H9BF4O | |
| Molar mass | 147.91 g·mol−1 |
| Melting point | 179.6–180 °C (355.3–356.0 °F; 452.8–453.1 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Trimethyloxonium tetrafluoroborate is the organic compound with the formula Me3OBF4. It is sometimes called the "Meerwein salt" after Hans Meerwein.) This salt is a strong methylating agent, being a source of CH3+. It is a white solid. Triethyloxonium tetrafluoroborate is a closely related reagent.
The compound is prepared by the reaction of boron trifluoride with dimethyl ether and epichlorohydrin:
The salt hydrolyzes readily:
Trimethyloxonium tetrafluoroborate is often ranked as the strongest reagent for the electrophilic methylation.
...
Wikipedia
