Tranexamic acid
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | \ˌtran-eks-ˌam-ik-\ |
| Trade names | Cyklokapron, others |
| AHFS/Drugs.com | FDA Professional Drug Information |
| Pregnancy category |
|
| Routes of administration |
by mouth, injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 34% |
| Biological half-life | 3.1 h |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.013.471 |
| Chemical and physical data | |
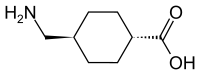
| Formula | C8H15NO2 |
| Molar mass | 157.21 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Tranexamic acid (TXA) is a medication used to treat or prevent excessive blood loss from major trauma, surgery, tooth removal, nose bleeds, and heavy menstruation. It is also used for hereditary angioedema. It is taken either by mouth or injection into a vein.
Side effects are rare. Some include changes in color vision, blood clots and allergic reactions. Greater caution is recommended in people with kidney disease. Tranexamic appears to be safe for use during pregnancy and breastfeeding. Tranexamic acid is in the antifibrinolytic family of medications.
Tranexamic acid was discovered in 1962 by Utako Okamoto. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Tranexamic acid is available as a generic medication. The wholesale cost in the developing world is about 4.38 to 4.89 USD for a course of treatment. In the United States a course of treatment costs 100 to 200 USD.
Tranexamic acid is frequently used following major trauma. Tranexamic acid is used to prevent and treat blood loss in a variety of situations, such as dental procedures for hemophiliacs, heavy menstrual bleeding, and surgeries with high risk of blood loss.
Tranexamic acid has been found to decrease the risk of death in people who have significant bleeding due to trauma. Its main benefit is if taken within the first three hours.
...
Wikipedia
