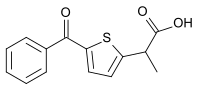
Tiaprofenic acid
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration |
oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Metabolism | 10% hepatic |
| Biological half-life | 1.5-2.5h |
| Excretion | 50-80% urine |
| Identifiers | |
|
|
| Synonyms | 5-Benzoyl-α-methyl-2-thiopheneacetic acid |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.046.649 |
| Chemical and physical data | |
| Formula | C14H12O3S |
| Molar mass | 260.309 |
| 3D model (Jmol) | |
| Chirality | Racemic mixture |
|
|
|
|
Tiaprofenic acid is a non-steroidal anti-inflammatory drug (NSAID) of the arylpropionic acid (profen) class, used to treat pain, especially arthritic pain. The typical adult dose is 300 mg twice daily. It is not recommended in children.
It is sparingly metabolised in the liver to two inactive metabolites. Most of the drug is eliminated unchanged in the urine. Renal disease impairs excretion, and it should be used with caution in renal disease.
Long-term use of tiaprofenic acid is associated with severe cystitis, roughly 100 times more commonly than other NSAIDs. It is contraindicated in patients with cystitis and urinary tract infections.
The earliest reports of clinical use are from France in 1975
It is marketed under the trade names Surgam, Surgamyl and Tiaprofen, and in generic formulations. A sustained-release preparation is available. It is an isomer of Suprofen.
...
Wikipedia
