Tafamidis
 |
|
| Clinical data | |
|---|---|
| Trade names | Vyndaqel |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
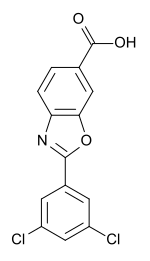
| Formula | C14H7Cl2NO3 |
| Molar mass | 308.116 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Tafamidis (INN, or Fx-1006A, trade name Vyndaqel) is a drug for the amelioration of transthyretin-related hereditary amyloidosis (also familial amyloid polyneuropathy, or FAP), a rare but deadly neurodegenerative disease. The drug was approved by the European Medicines Agency in November 2011 and by the Japanese Pharmaceuticals and Medical Devices Agency in September 2013.
The marketed drug, a meglumine salt, has completed an 18 month placebo controlled phase II/III clinical trial, and an 12 month extension study which provides evidence that tafamidis slows progression of Familial amyloid polyneuropathy. Tafamidis (20 mg once daily) is used in adult patients with an early stage (stage 1) of familial amyloidotic polyneuropathy.
Tafamidis was discovered in the Jeffery W. Kelly Laboratory at The Scripps Research Institute using a structure-based drug design strategy and was developed at FoldRx pharmaceuticals, a biotechnology company Kelly co-founded with Susan Lindquist. FoldRx was led by Richard Labaudiniere when it was acquired by Pfizer in 2010.
Tafamidis functions by kinetic stabilization of the correctly folded tetrameric form of the transthyretin (TTR) protein. In patients with FAP, this protein dissociates in a process that is rate limiting for aggregation including amyloid fibril formation, causing failure of the autonomic nervous system and/or the peripheral nervous system (neurodegeneration) initially and later failure of the heart. Kinetic Stabilization of tetrameric transthyretin in familial amyloid polyneuropathy patients provides the first pharmacologic evidence that the process of amyloid fibril formation causes this disease, as treatment with tafamidis dramatically slows the process of amyloid fibril formation and the degeneration of post-mitotic tissue. Sixty % of the patients enrolled in the initial clinical trial have the same or an improved neurologic impairment score after six years of taking tafamidis, whereas 30% of the patients progress at a rate ≤ 1/5 of that predicted by the natural history. Importantly, all of the V30M FAP patients remain stage 1 patients after 6 years on tafamidis out of four stages of disease progression. [Data presented orally by Professor Coelho in Brazil in 2013]
...
Wikipedia
