Swainsonine
 |
|
| Names | |
|---|---|
|
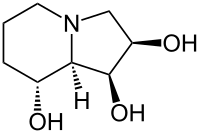
IUPAC name
(1S,2R,8R,8aR)-1,2,3,5,6,7,8,8a-Octahydroindolizine-1,2,8-triol
|
|
| Other names
Tridolgosir
|
|
| Identifiers | |
|
72741-87-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:9367 |
| ChEMBL |
ChEMBL371197 |
| ChemSpider |
46788 |
| DrugBank |
DB02034 |
| ECHA InfoCard | 100.123.531 |
| KEGG |
C10173 |
| PubChem | 51683 |
| UNII |
RSY4RK37KQ |
|
|
|
|
| Properties | |
| C8H15NO3 | |
| Molar mass | 173.2 |
| Melting point | 143 to 144 °C (289 to 291 °F; 416 to 417 K) |
| 10 mg/1 mL | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Swainsonine is an indolizidine alkaloid. It is a potent inhibitor of Golgi alpha-mannosidase II, an immunomodulator, and a potential chemotherapy drug. As a toxin in locoweed (likely its primary toxin) it also is a significant cause of economic losses in industries, particularly in North America.
Swainsonine inhibits glycoside hydrolases, specifically N-linked glycosylation. Disruption of Golgi alpha-mannosidase II with swainsonine induces hybrid-type glycans. These glycans have a Man5GlcNAc2 core with processing on the 3-arm that resembles so-called complex-type glycans.
The pharmacological properties of this product have not been fully investigated.
Swainsonine is a natural product that has been isolated from numerous species of flowering plants and some fungi (see Locoweed). It was first isolated from Swainsona in Australia.
Swainsonine is extracted commercially from several species of plants and fungi, including the soil fungus Metarhizium anisopliae. It also can be produced from total synthesis.
The biosynthesis of swainsonine has been investigated in the fungus Rhizoctonia leguminicola, and it initially involves the conversion of lysine into pipecolic acid. The pyrrolidine ring is then formed via retention of the carbon atom of the pipecolate's carboxyl group, as well as the coupling of two more carbon atoms from either acetate or malonate to form a pipecolylacetate. The retention of the carboxyl carbon is striking, since it is normally lost in the biosynthesis of most other alkaloids.
...
Wikipedia
