Succinate - coenzyme Q reductase
| succinate dehydrogenase (succinate-ubiquinone oxidoreductase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
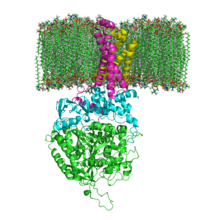
The structure of SQR in a phospholipid membrane. SdhA, SdhB, SdhC and SdhD
|
|||||||||
| Identifiers | |||||||||
| EC number | 1.3.5.1 | ||||||||
| CAS number | 9028-11-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
Succinate dehydrogenase or succinate-coenzyme Q reductase (SQR) or respiratory Complex II is an enzyme complex, found in many bacterial cells and in the of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain.
In step 6 of the citric acid cycle, SQR catalyzes the oxidation of succinate to fumarate with the reduction of ubiquinone to ubiquinol. This occurs in the inner mitochondrial membrane by coupling the two reactions together.
and many bacterial monomer SQRs are composed of four subunits: two hydrophilic and two hydrophobic. The first two subunits, a flavoprotein (SdhA) and an iron-sulfur protein (SdhB), are hydrophilic. SdhA contains a covalently attached flavin adenine dinucleotide (FAD) cofactor and the succinate binding site and SdhB contains three iron-sulfur clusters: [2Fe-2S], [4Fe-4S], and [3Fe-4S]. The second two subunits are hydrophobic membrane anchor subunits, SdhC and SdhD. Human mitochondria contain two distinct isoforms of SdhA (Fp subunits type I and type II), these isoforms are also found in Ascaris suum and Caenorhabditis elegans. The subunits form a membrane-bound complex with six transmembrane helices containing one heme b group and a ubiquinone-binding site. Two phospholipid molecules, one cardiolipin and one phosphatidylethanolamine, are also found in the SdhC and SdhD subunits (not shown in the image). They serve to occupy the hydrophobic space below the heme b. These subunits are displayed in the attached image. SdhA is green, SdhB is teal, SdhC is fuchsia, and SdhD is yellow. Around SdhC and SdhD is a phospholipid membrane with the intermembrane space at the top of the image.
...
Wikipedia
