Solanine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
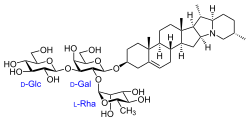
(2S,3R,4S,5S,6R)-2-(((2R,3S,4S,5R,6R)-3-hydroxy-2-(hydroxymethyl)-5-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6-(((4S,6aR,6bS,8aS,8bR,9S,9aR,14aS,15aS,15bS)-6a,8a,9-trimethyl-3,4,5,6,6a,6b,7,8,8a,8b,9,9a,10,11,12,13,14a,15,15a,15b-icosahydro-1H-naphtho[2',1':4,5]indeno[1,2-b]indolizin-4-yl)oxy)tetrahydro-2H-pyran-4-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
|
|
| Identifiers | |
|
20562-02-1 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:9188 |
| ChemSpider |
28033 |
| ECHA InfoCard | 100.039.875 |
| PubChem | 6537493 |
| UNII |
3FYV8328OK |
|
|
|
|
| Properties | |
| C45H73NO15 | |
| Molar mass | 868.06 |
| Appearance | white crystalline solid |
| Melting point | 271 to 273 °C (520 to 523 °F; 544 to 546 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Solanine is a glycoalkaloid poison found in species of the nightshade family (Solanaceae), such as the potato (Solanum tuberosum), the tomato (Solanum lycopersicum), and the eggplant (Solanum melongena). It can occur naturally in any part of the plant, including the leaves, fruit, and tubers. Solanine has pesticidal properties, and it is one of the plant's natural defenses. Solanine was first isolated in 1820 from the berries of the European black nightshade (Solanum nigrum), after which it was named.
Solanine poisoning is primarily displayed by gastrointestinal and neurological disorders. Symptoms include nausea, diarrhea, vomiting, stomach cramps, burning of the throat, cardiac dysrhythmia, nightmares, headache and dizziness. In more severe cases, hallucinations, loss of sensation, paralysis, fever, jaundice, dilated pupils, hypothermia, and death have been reported.
Ingestion of solanine in moderate amounts can cause death. One study suggests that doses of 2 to 5 mg/kg of body weight can cause toxic symptoms, and doses of 3 to 6 mg/kg of body weight can be fatal.
Symptoms usually occur 8 to 12 hours after ingestion, but may occur as rapidly as 10 minutes after eating high-solanine foods.
Solanum glycoalkaloids can inhibit cholinesterase, disrupt cell membranes, and cause birth defects. One study suggests that the toxic mechanism of solanine is caused by the chemical's interaction with membranes. Experiments show that solanine exposure opens the potassium channels of mitochondria, decreasing their membrane potential. This, in turn, leads to K+ being transported from the mitochondria into the cytoplasm, and this increased concentration of K+ in the cytoplasm triggers cell damage and apoptosis.
...
Wikipedia
