Snake venoms
| Snake toxin | |||||||||
|---|---|---|---|---|---|---|---|---|---|

Vipera berus - Venom delivery apparatus
|
|||||||||
| Identifiers | |||||||||
| Symbol | Toxin_1 | ||||||||
| Pfam | PF00087 | ||||||||
| InterPro | IPR003571 | ||||||||
| PROSITE | PDOC00245 | ||||||||
| SCOP | 2ctx | ||||||||
| SUPERFAMILY | 2ctx | ||||||||
| OPM superfamily | 55 | ||||||||
| OPM protein | 1txa | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
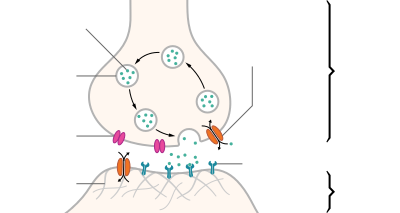
| Structure of a typical chemical synapse |
|---|
Snake venom is highly modified saliva containing zootoxins which facilitates the immobilization and digestion of prey, and defends against threats. It is injected by unique fangs after a bite, and some species are also able to spit.
The glands that secrete the zootoxins are a modification of the parotid salivary gland found in other vertebrates, and are usually situated on each side of the head, below and behind the eye, and encapsulated in a muscular sheath. The glands have large alveoli in which the synthesized venom is stored before being conveyed by a duct to the base of channeled or tubular fangs through which it is ejected.
Venoms contain more than 20 different compounds, mostly proteins and polypeptides. A complex mixture of proteins, enzymes, and various other substances with toxic and lethal properties serves to immobilize the prey animal, enzymes play an important role in the digestion of prey, and various other substances are responsible for important but non-lethal biological effects. Some of the proteins in snake venom have very specific effects on various biological functions including blood coagulation, blood pressure regulation, and transmission of the nervous or muscular impulse, and have been developed for use as pharmacological or diagnostic tools, and even useful drugs.
Charles Lucien Bonaparte, the son of Lucien Bonaparte, younger brother of Napoleon Bonaparte, was the first to establish the proteinaceous nature of snake venom in 1843.
Proteins constitute 90-95% of venom's dry weight and they are responsible for almost all of its biological effects. Among hundreds, even thousands of proteins found in venom, there are toxins, neurotoxins in particular, as well as nontoxic proteins (which also have pharmacological properties), and many enzymes, especially hydrolytic ones. Enzymes (molecular weight 13-150 KDa) make-up 80-90% of viperid and 25-70% of elapid venoms: digestive hydrolases, L-amino acid oxidase, phospholipases, thrombin-like pro-coagulant, and kallikrein-like serine proteases and metalloproteinases (hemorrhagins), which damage vascular endothelium. Polypeptide toxins (molecular weight 5-10 KDa) include cytotoxins, cardiotoxins, and postsynaptic neurotoxins (such as α-bungarotoxin and α-Cobratoxin), which bind to acetylcholine receptors at neuromuscular junctions. Compounds with low molecular weight (up to 1.5 KDa) include metals, peptides, lipids, nucleosides, carbohydrates, amines, and oligopeptides, which inhibit angiotensin converting enzyme (ACE) and potentiate bradykinin (BPP). Inter- and intra-species variation in venom chemical composition is geographical and ontogenic.Phosphodiesterases interfere with the prey's cardiac system, mainly to lower the blood pressure. Phospholipase A2 causes hemolysis by lysing the phospholipid cell membranes of red blood cells. Amino acid oxidases and proteases are used for digestion. Amino acid oxidase also triggers some other enzymes and is responsible for the yellow colour of the venom of some species. Hyaluronidase increases tissue permeability to accelerate absorption of other enzymes into tissues. Some snake venoms carry fasciculins, like the mambas (Dendroaspis), which inhibit cholinesterase to make the prey lose muscle control.
...
Wikipedia