Santonin
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
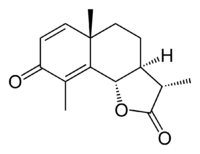
(3S,3aS,5aS,9bS)-3,5a,9-trimethyl-3a,5,5a,9b-tetrahydronaphtho[1,2-b]furan-2,8(3H,4H)-dione
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.874 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C15H18O3 | |
| Molar mass | 246.30162 |
| Melting point | 172 °C (342 °F; 445 K) |
| Boiling point | 423 °C (793 °F; 696 K) |
| insoluble | |
| Vapor pressure | 1*10−7mmHg |
| Hazards | |
| Flash point | 190 °C (374 °F; 463 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Santonin is a drug which was widely used in the past as an anthelminthic, a drug that expels parasitic worms (helminths) from the body, by either killing or stunning them. Santonin was formerly listed in U.S. and British pharmacopoeia but has fallen out of use with the development of safer ascaricides and is no longer registered as a drug in most countries.
Santonin can be converted to santonic acid (C15H20O4) via based-catalyzed hydrolysis followed by a multistep rearrangement process.
Santonin dissolves in alkalies with formation of salts of this carboxylic acid. Santonin, in acetic acid solution, when exposed to sunlight for about a month, is converted into (colorless) photosantonic acid (C15H22O5) which is generally regarded as less toxic. The ethyl ester of the latter is obtained when an alcoholic solution of santonin is exposed to sunlight (Sestini). A yellow coloration is developed upon exposure of santonin to light. Santonin is optically levorotatory.
Santonin is an organic chemical consisting of colorless flat prisms, turning slightly yellow from the action of light and soluble in alcohol, chloroform and boiling water. It is derived from santonica (the unexpanded flower-heads of Artemisia maritima var. stechmanniana). Others refer to A. cina or A. chamaemelifolia as being the derivative species.
...
Wikipedia
