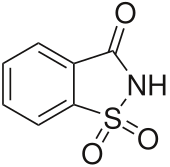
Saccharine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
2H-1λ6,2-Benzothiazol-1,1,3-trione
|
|
| Other names
Benzoic sulfimide
|
|
| Identifiers | |
|
81-07-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:32111 |
| ChEMBL |
ChEMBL310671 |
| ChemSpider |
4959 |
| ECHA InfoCard | 100.001.202 |
| E number | E954 (glazing agents, ...) |
| 5432 | |
| KEGG |
D01085 |
| PubChem | 5143 |
| UNII |
FST467XS7D |
|
|
|
|
| Properties | |
| C7H5NO3S | |
| Molar mass | 183.18 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 0.828 g/cm3 |
| Melting point | 228.8 to 229.7 °C (443.8 to 445.5 °F; 501.9 to 502.8 K) |
| 1 g per 290 mL | |
| Acidity (pKa) | 1.6 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Saccharin is an artificial sweetener with effectively no food energy that is about 300–400 times as sweet as sucrose or table sugar, but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products such as drinks, candies, cookies, medicines, and toothpaste.
Saccharin derives its name from the word "saccharine", meaning "sugary". The word saccharine is used figuratively, often in a derogative sense, to describe something "unpleasantly over-polite" or "overly sweet". Both words are derived from the Greek word σάκχαρον (sakcharon) meaning "gravel".
Saccharin is heat stable. It does not react chemically with other food ingredients, as such, it stores well. Blends of saccharin with other sweeteners are often used to compensate for each sweetener's weaknesses and faults. A 10:1 cyclamate:saccharin blend is common in countries where both these sweeteners are legal; in this blend, each sweetener masks the other's off taste. Saccharin is often used with aspartame in diet carbonated soft drinks, so some sweetness remains should the fountain syrup be stored beyond aspartame's relatively short shelf life.
In its acid form, saccharin is not water-soluble. The form used as an artificial sweetener is usually its sodium salt. The calcium salt is also sometimes used, especially by people restricting their dietary sodium intake. Both salts are highly water-soluble: 0.67 g/ml water at room temperature.
In the 1970s, studies performed on laboratory rats found an association between consumption of high doses of saccharin and the development of bladder cancer. However, further study determined that this effect was due to a mechanism that is not relevant to humans.Epidemiological studies have shown no evidence that saccharin is associated with bladder cancer in humans. The International Agency for Research on Cancer (IARC) originally classified saccharin in Group 2B ("possibly carcinogenic to humans") based on the rat studies, but downgraded it to Group 3 ("not classifiable as to the carcinogenicity to humans") upon review of the subsequent research.
...
Wikipedia
