Ruthenium tetroxide
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Ruthenium(VIII) oxide
|
|||
| Identifiers | |||
| ECHA InfoCard | 100.039.815 | ||
|
PubChem CID
|
|||
| Properties | |||
| RuO4 | |||
| Molar mass | 165.07 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | pungent | ||
| Density | 3.29 g/cm3 | ||
| Melting point | 25.4 °C (77.7 °F; 298.5 K) | ||
| Boiling point | 40.0 °C (104.0 °F; 313.1 K) | ||
| 2% w/v at 20 °C | |||
| Solubility in other solvents | Soluble in Carbon tetrachloride Chloroform |
||
| Structure | |||
| tetrahedral | |||
| zero | |||
| Hazards | |||
| Safety data sheet | external MSDS sheet | ||
| NFPA 704 | |||
| Related compounds | |||
|
Related compounds
|
RuO2 RuCl3 |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
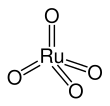
Ruthenium tetroxide (Ruthenium(VIII) oxide) is the inorganic compound with the formula RuO4. It is a colourless, diamagnetic liquid, but samples are typically black due to impurities. It is volatile. The analogous OsO4 is more widely used and better known. One of the few solvents in which it forms stable solutions is CCl4.
RuO4 is prepared by oxidation of ruthenium(III) chloride with NaIO4.
In typical reactions featuring RuO4 as the oxidant, many forms of ruthenium usefully serve as precursors to RuO4, such as oxide hydrates or hydrated chloride.
The molecule adopts a tetrahedral geometry, with the Ru-O distances ranging from 169 to 170 pm.
The main value of RuO4 is as an intermediate in the production of ruthenium compounds and metal from ores. Like other platinum group metals (PGMs), ruthenium occurs at low concentrations and often mixed with other PGMs. Together with OsO4, it is separated from other PGMs by distillation of a chlorine-oxidized extract. Ruthenium is separated from OsO4 by reducing RuO4 with hydrochloric acid, a process that exploits the highly positive reduction potential for the [RuO4]0/- couple.
RuO4 is only of specialized value in organic chemistry because it oxidizes virtually any hydrocarbon. For example, it will oxidize adamantane to 1-adamantanol. Because it is such an aggressive oxidant, reaction conditions must be mild, generally room temperature. Although a strong oxidant, RuO4 oxidations do not perturb stereocenters that are not oxidized. Illustrative is the oxidation of the following diol to a carboxylic acid:
Oxidation of epoxy alcohols also occurs without degradation of the epoxide ring:
...
Wikipedia