Rubidium chloride
 |
|
 |
|
| Names | |
|---|---|
| Other names
rubidium(I) chloride
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.310 |
|
PubChem CID
|
|
| RTECS number | VL8575000 |
| UNII | |
|
|
|
|
| Properties | |
| RbCl | |
| Molar mass | 120.921 g/mol |
| Appearance | white crystals hygroscopic |
| Density | 2.80 g/cm3 (25 °C) 2.088 g/mL (750 °C) |
| Melting point | 718 °C (1,324 °F; 991 K) |
| Boiling point | 1,390 °C (2,530 °F; 1,660 K) |
| 77 g/100mL (0 °C) 91 g/100 mL (20 °C) 130 g/100 mL (100 °C) |
|
| Solubility in methanol | 1.41 g/100 mL |
| −46.0·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.5322 |
| Thermochemistry | |
| 52.4 J K−1 mol−1 | |
|
Std molar
entropy (S |
95.9 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−435.14 kJ/mol |
| Hazards | |
| Safety data sheet | Fisher Scientific |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
4440 mg/kg (rat) |
| Related compounds | |
|
Other anions
|
Rubidium fluoride Rubidium bromide Rubidium iodide Rubidium astatide |
|
Other cations
|
Lithium chloride Sodium chloride Potassium chloride Caesium chloride Francium chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Rubidium chloride is the chemical compound with the formula RbCl. This alkali metal halide is composed of rubidium and chlorine, and finds diverse uses ranging from electrochemistry to molecular biology.
In its gas phase, RbCl is diatomic with a bond length estimated at 2.7868 Å. This distance increases to 3.285 Å for cubic RbCl, reflecting the higher coordination number of the ions in the solid phase.
Depending on conditions, solid RbCl exists in one of three arrangements or polymorphs as determined with holographic imaging:
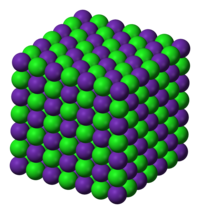
The sodium chloride (NaCl) polymorph is most common. A cubic close-packed arrangement of chloride anions with rubidium cations filling the octahedral holes describes this polymorph. Both ions are six-coordinate in this arrangement. This polymorph's lattice energy is only 3.2 kJ/mol less than the following structure's.
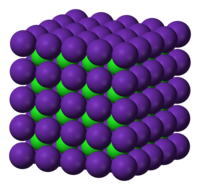
At high temperature and pressure, RbCl adopts the caesium chloride (CsCl) structure (NaCl and KCl undergo the same structural change at high pressures). Here, the chloride ions form a simple cubic arrangement with chloride anions occupying the vertices of a cube surrounding a central Rb+. This is RbCl's densest packing motif. Because a cube has eight vertices, both ions' coordination numbers equal eight. This is RbCl's highest possible coordination number. Therefore, according to the radius ratio rule, cations in this polymorph will reach their largest apparent radius because the anion-cation distances are greatest.
The sphalerite polymorph of rubidium chloride is extremely rare, resulting in few structural studies. The lattice energy, however, for this formation is predicted to nearly 40.0 kJ/mol smaller than those of the preceding structures.
The most common preparation of pure rubidium chloride involves the reaction of its hydroxide with hydrochloric acid, followed by recrystallization:
...
Wikipedia

