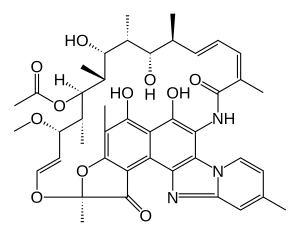
Rifaximin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Xifaxan, Xifaxanta, Normix, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604027 |
| Pregnancy category |
|
| Routes of administration |
by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | < 0.4% |
| Metabolism | Hepatic |
| Biological half-life | 6 hours |
| Excretion | Fecal (97%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.111.624 |
| Chemical and physical data | |
| Formula | C43H51N3O11 |
| Molar mass | 785.879 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Rifaximin is an antibiotic used to treat traveler's diarrhea, irritable bowel syndrome, and hepatic encephalopathy. It has poor absorption when taken by mouth.
It is based on rifamycin. Rifaximin was approved for medical use in the United States in 2004. In the United States it costs 62.13 USD per day for 1100 mg of rifaximin (1,864.00 USD per month) as of January 2017. In Russia as of 2016 a similar dose costs 231.25 RUB (approximately 4 USD as of 2016 exchange rate).
Rifaximin may be used to treat and prevent traveler's diarrhea.
It may be efficacious in relieving chronic functional symptoms of bloating and flatulence that are common in irritable bowel syndrome (IBS),
Rifaximin may also be a useful addition to vancomycin when treating patients with relapsing C. difficile infections. Although exposure to rifamycins in the past may increase risk for resistance, so rifaximin should be avoided in such cases.
In the United States, rifaximin has orphan drug status for the treatment of hepatic encephalopathy. Although high-quality evidence is still lacking, rifaximin appears to be as effective as or more effective than other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster. Rifaximin is taken by mouth. It has minimal side effects, prevents reoccurring encephalopathy, and is associated with high patient satisfaction. Patients are more compliant and satisfied to take this medication than any other due to minimal side effects, prolong remission, and overall cost. The drawbacks to rifaximin are increased cost and lack of robust clinical trials for HE without combination lactulose therapy.
Rifaximin interferes with transcription by binding to the β-subunit of bacterial RNA polymerase. This results in the blockage of the translocation step that normally follows the formation of the first phosphodiester bond, which occurs in the transcription process.
...
Wikipedia
