Ribose sugar
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
(2S,3R,4S,5R)-5-(hydroxymethyl)oxolane-2,3,4-triol
|
|||
| Other names
D-Ribose
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|
||
| ChemSpider |
|
||
| DrugBank | |||
| ECHA InfoCard | 100.000.055 | ||
| EC Number | 200-059-4 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C5H10O5 | |||
| Molar mass | 150.13 g/mol | ||
| Appearance | white solid | ||
| Melting point | 95 °C (203 °F; 368 K) | ||
| 100 g/1 L (25 °C (77 °F)) | |||
|
Chiral rotation ([α]D)
|
−21.5° (H2O) | ||
| Related compounds | |||
|
Related aldopentoses
|
Arabinose Xylose Lyxose |
||
|
Related compounds
|
Deoxyribose | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
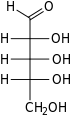
Ribose is a carbohydrate with the formula C5H10O5; specifically, it is a pentose monosaccharide (simple sugar) with linear form H−(C=O)−(CHOH)4−H, which has all the hydroxyl groups on the same side in the Fischer projection.
The term may refer to either of two enantiomers. The term usually indicates D-ribose, which occurs widely in nature and is discussed here. Its synthetic mirror image, L-ribose, is not found in nature.
D-Ribose was first reported in 1891 by Emil Fischer. It is a C'-2 carbon epimer of the sugar D-arabinose (both isomers of which are named for their source, gum arabic) and ribose itself is named as a partial rearrangement of letters in the word 'arabinose'.
The ribose β-D-ribofuranose forms part of the backbone of RNA. It is related to deoxyribose, which is found in DNA. Phosphorylated derivatives of ribose such as ATP and NADH play central roles in metabolism. cAMP and cGMP, formed from ATP and GTP, serve as secondary messengers in some signalling pathways.
Ribose is an aldopentose (a monosaccharide containing five carbon atoms) that, in its open chain form, has an aldehyde functional group at one end. In the conventional numbering scheme for monosaccharides, the carbon atoms are numbered from C1' (in the aldehyde group) to C5'. The deoxyribose derivative found in DNA differs from ribose by having a hydrogen atom in place of the hydroxyl group at C2'. This hydroxyl group performs a function in RNA splicing.
...
Wikipedia