Rhodium(III) chloride
 |
|
 |
|
| Names | |
|---|---|
| Other names
Rhodium trichloride
|
|
| Identifiers | |
|
|
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.138 |
| EC Number | 233-165-4 |
|
PubChem CID
|
|
| RTECS number | VI9290000 |
| UNII | |
|
|
|
|
| Properties | |
| RhCl3 | |
| Molar mass | 209.26 g/mol |
| Appearance | dark red solid deliquescent |
| Density | 5.38 g/cm3, solid |
| Melting point | ca. 450 °C (842 °F; 723 K) |
| Boiling point | 717 °C (1,323 °F; 990 K) |
| insoluble | |
| Solubility | soluble in hydroxide and cyanide solutions, also soluble in aqua regia |
| Acidity (pKa) | acidic in solution |
| −-7.5·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| octahedral | |
| Thermochemistry | |
|
Std enthalpy of
formation (ΔfH |
−234 kJ/mol |
| Hazards | |
| Safety data sheet | ICSC 0746 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
>500 mg/kg (rat, oral) 1302 mg/kg (rat, oral) |
| Related compounds | |
|
Other anions
|
Rhodium(III) fluoride Rhodium(III) bromide Rhodium(III) iodide |
|
Other cations
|
Cobalt(II) chloride Iridium(III) chloride |
|
Related compounds
|
Ruthenium(III) chloride Palladium(II) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid.
Hydrated rhodium trichloride usually refers to compound with the approximate formula RhCl3(H2O)3.103Rh NMR spectroscopy shows that solutions of this material consist of several species, the proportions of which change with time and depend on the concentration of chloride. The relative distribution of these species determines the colour of the solutions, which can range from yellow (the hexaaquo ion) to "raspberry-red". Some of these species are [Rh(H2O)6]3+, [RhCl(H2O)5]2+, cis- and trans-[RhCl2(H2O)4]+, and [RhCl3(H2O)3]. Individual ions have been separated by ion exchange chromatography.
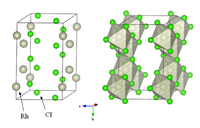
Anhydrous rhodium chloride crystallises with a monoclinic crystal structure, as in YCl3 and AlCl3. It is a dense brown solid that is insoluble in common solvents and of little value in the laboratory.
RhCl3(H2O)3 is produced from salts such as Na3RhCl6, the latter being obtained in the purification of rhodium from the other platinum group metals such as platinum and iridium. The sodium salt is converted to H3RhCl6 by ion exchange chromatography. Recrystallization of this acidic salt from water affords the hydrated trichloride, sometimes called "soluble rhodium trichloride." Anhydrous RhCl3 is prepared by reaction of chlorine with rhodium sponge metal at 200–300 °C. Above 800 °C, the anhydrous chloride reverts to Rh metal and chlorine.
...
Wikipedia
