Retigabine
 |
|
| Clinical data | |
|---|---|
| Trade names | Trobalt, Potiga |
| AHFS/Drugs.com | potiga |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 60–80% |
| Metabolism | Hepatic glucuronidation and acetylation. not involved |
| Biological half-life | 8 hours (mean), range: 7–11 hours |
| Excretion | Renal (84%) |
| Identifiers | |
|
|
| Synonyms | D-23129 |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.158.123 |
| Chemical and physical data | |
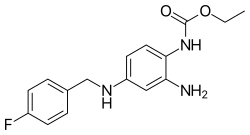
| Formula | C16H18FN3O2 |
| Molar mass | 303.331 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production will be discontinued after June 2017, and the product will no longer be commercially available.
Retigabine works primarily as a potassium channel opener—that is, by activating a certain family of voltage-gated potassium channels in the brain. This mechanism of action is unique among antiepileptic drugs, and may hold promise for the treatment of other neurologic conditions, including tinnitus, migraine and neuropathic pain.
The adverse effects found in the Phase II trial mainly affected the central nervous system, and appeared to be dose-related. The most common adverse effects were drowsiness, dizziness, tinnitus and vertigo, confusion, and slurred speech. Less common side effects included tremor, memory loss, gait disturbances, and double vision. In 2013 FDA warned the public that, Potiga (ezogabine) can cause blue skin discoloration and eye abnormalities characterized by pigment changes in the retina. FDA does not currently know if these changes are reversible. FDA is working with the manufacturer to gather and evaluate all available information to better understand these events. FDA will update the public when more information is available. Psychiatric symptoms and difficulty urinating have also been reported, with most cases occurring in the first 2 months of treatment.
...
Wikipedia
