Remifentanil
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Ultiva |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not applicable (intravenous administration) |
| Protein binding | 70% (bound to plasma proteins) |
| Metabolism | cleaved by non-specific plasma and tissue esterases |
| Biological half-life | 1-20 minutes |
| Identifiers | |
|
|
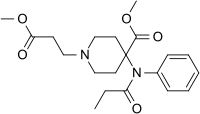
| Synonyms | methyl 1-(2-methoxycarbonylethyl)-4-(phenyl-propanoyl-amino)-piperidine-4-carboxylate |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.211.201 |
| Chemical and physical data | |
| Formula | C20H28N2O5 |
| Molar mass | 376.447 g/mol |
| 3D model (Jmol) | |
| Melting point | 5 °C (41 °F) |
|
|
|
|
Remifentanil is a potent, short-acting synthetic opioid analgesic drug. It is given to patients during surgery to relieve pain and as an adjunct to an anaesthetic. Remifentanil is used for sedation as well as combined with other medications for use in general anesthesia. The use of remifentanil has made possible the use of high-dose opioid and low-dose hypnotic anesthesia, due to synergism between remifentanil and various hypnotic drugs and volatile anesthetics.
Remifentanil is used as an opioid analgesic that has a rapid onset and rapid recovery time. It has been used effectively during craniotomies, spinal surgery,cardiac surgery, and gastric bypass surgery. While opiates function similarly, with respect to analgesia, the pharmacokinetics of remifentanil allows for quicker post-operative recovery.
It is administered in the form remifentanil hydrochloride and in adults is given as an intravenous infusion in doses ranging from 0.1 microgram per kilogram per minute to 0.5 (µg/kg)/min. Children may require higher infusion rates (up to 1.0 (µg/kg)/min). The clinically useful infusion rates are 0.025-0.1 (µg/kg)/min for sedation (rates adjusted to age of patient, severity of their illness and invasiveness of surgical procedure). Small amounts of other sedative medications are usually co-administered with remifentanil to produce sedation. Clinically useful infusion rates in general anesthesia vary but are usually 0.1-1 µg/kg/min.
Remifentanil can be administered as part of an anesthesia technique called TIVA (Total Intravenous Anesthesia) using computer controlled infusion pumps in a process called target controlled infusion or TCI. A target plasma concentration is entered as ng/ml into the pump, which calculates its infusion rate according to patient factors like age and weight. Induction levels of 4 ng/ml are commonly used, but it generally varies between 3-8 ng/ml. For certain surgical procedures that produce particularly strong stimuli a level of up to 15 ng/ml might be needed. The relatively short context-sensitive half-life of Remifentanil allows the desired blood plasma level to be achieved quickly and also for the same reason, recovery occurs quickly.
...
Wikipedia
